Preprint
Review
Ten-Year Observational Study of Patients with Lung Adenocarcinoma: Clinical Outcomes, Prognostic Factors, and Five-Year Survival Rates
Altmetrics
Downloads
66
Views
37
Comments
0
This version is not peer-reviewed
Submitted:
21 September 2024
Posted:
23 September 2024
You are already at the latest version
Alerts
Abstract
Background/Objectives: Lung carcinoma is the leading cause of cancer-related deaths globally, with lung adenocarcinoma being the most prevalent subtype. This study aims to review the clinical data and survival outcomes of patients diagnosed with lung adenocarcinoma who underwent surgical treatment. Methods: We conducted a retrospective analysis of 471 patients who had surgery for lung adenocarcinoma at our institution. Their medical histories, including previous neoplasms, comorbidities, tumor characteristics, and symptoms, were thoroughly reviewed. We calculated the overall survival rate and evaluated the impact of tumor grading and spread through air spaces (STAS) on patient outcomes. Results: The survival rate for the entire cohort was 76.23%. There was no significant difference in survival rates between patients classified as G1 and G2, suggesting that a simplified two-tier grading system may be more effective. Patients with STAS had a lower survival rate than those without STAS. Conclusions: Our findings indicate that a simplified grading system may improve prognostic evaluations for lung adenocarcinoma patients. Furthermore, STAS is a crucial factor affecting survival rates and should be considered in future treatment strategies. Expanding research in this area is essential to enhance treatment approaches for lung adenocarcinoma patients.
Keywords:
Subject: Medicine and Pharmacology - Pathology and Pathobiology
1. Introduction
Lung carcinoma remains one of the most prevalent and lethal forms of carcinoma worldwide, accounting for a significant number of cancer-related deaths each year [1,2]. According to GLOBOCAN 2020 statistics, there were 1.8 million reported deaths and 2.2 million new cases in 2020 [2]. Generally, lung carcinoma can be categorized into small cell lung carcinoma and non-small cell lung carcinoma (NSCLC), with the latter accounting for over 80% of cases [3,4,5]. Within NSCLC, the following main histological types can be distinguished: squamous carcinoma, adenocarcinoma, and large cell carcinoma [6].
The primary risk factor remains exposure to tobacco smoke, which has been linked to as much as 80-90% of lung carcinoma cases [7,8,9]. Additionally, exposure to arsenates, nitrosamines and asbestos is associated with lung carcinoma etiology [7]. Genetic factors and air pollution can also contribute to the development of lung carcinoma [7].
Due to the nonspecific nature of early clinical symptoms, over 60% of NSCLC patients are already in the middle or advanced stages of the disease at the time of diagnosis [4,8,10].
Among its various subtypes, adenocarcinoma of the lung has emerged as the most common histological type, representing a distinct challenge in terms of diagnosis, treatment, and patient care [11]. Its 5-year overall survival rate remains approximately 15% [4,6].
The aim of this study was to provide a thorough overview of patients with lung adenocarcinoma treated surgically, exploring their complete disease history and survival data.
2. Materials and Methods
2.1. Study Design
A study was conducted on a cohort of 471 patients with adenocarcinoma of the lung out of total 1051 patients who underwent radical anatomical resection of lung tissue (lobectomy, bilobectomy, or pneumonectomy) due to lung carcinoma between May 2012 and December 2022. A detailed analysis of medical records for all patients operated on for lung carcinoma at our center was performed and collected in a dedicated database. This database included information on prior medical history, exposure to environmental hazards and stimulants, family cancer history, precise cancer stage assessment, exact lung cancer type diagnosis determined through postoperative histopathological examination, and perioperative care results. Additionally, each patient who underwent surgery was subsequently monitored through our outpatient clinic, enabling the evaluation of long-term treatment outcomes. The day of the surgical procedure marked the starting point of the observation period, which extended up to five years post-surgery. Survival data for patients were gathered until May 1, 2022, with all subsequent outcomes considered incomplete.
The inclusion criteria: histopathologically confirmed primary adenocarcinoma.
The exclusion criteria: histopathologically confirmed carcinoma other than adenocarcinoma, secondary lung neoplasms confirmed histopathologically, specific types of adenocarcinomas (colloid/fetal/enteric-type), and the occurrence of more than one histologically distinct tumor in post-operative material.
The mean age of patients in the cohort was 65.9 ± 7.81 years (range 38-86 years). There were 252 women (53.50%) and 219 men (46.50%). In total, 115 of the patients were non-smokers (24.42%, 95% CI: 20.54–28.30%). Further details regarding the group are presented in the results section. The study received approval from the Bioethics Committee of the Medical University of Silesia in Katowice.
2.2. Statistical Analysis
The data were presented as the number of cases with percentage values for categorical variables and the mean ± SD for quantitative variables. The normality assumption of each quantitative variable was evaluated through graphical interpretation of Q–Q plots and histograms. The Kaplan–Meier method was used to determine the survival probability among groups. In instances where comparisons encompassed more than two groups, the Log-rank test with Mantel correction was utilized. To assess the influence of more than one variable on patients’ survival, the Cox proportional hazard model was performed. p-values lower than 0.05 were considered significant. Analysis was performed using the R language in RStudio software.
3. Results
3.1. General Characteristics of the Study Group
The mean age within the study group was 65.9 years. The percentage of women was slightly higher than men, 53.5% compared to 46.5%. Over 75.5% of the patients were smokers (Table 1).
3.2. History of Previous Neoplastic Diseases
15.89% of patients had a history of former cancer. The most common malignancies among them were breast, prostate, and bladder carcinomas (Table 2).
3.3. Comorbidities among Patients
The most common comorbidities among our patients were hypertension (54.99%), coronary disease (18.47%), non-insulin-dependent diabetes mellitus (16.99%), and COPD (15.50%) (Table 3).
3.4. Symptoms of Lung Adenocarcinoma
Over 60% of the patients presented with some sort of symptoms. The most common symptom of lung adenocarcinoma was cough, which occurred in 28.24% of the study group members (Table 4).
3.5. Tumor Characteristics in the Study Group
Around 50% of the patients exhibited pT1 tumors, while pT2 tumors were observed in approximately 34% of the cases. Most of the patients (84.71%) had no involvement of regional lymph nodes, and none of the patients suffered from distant metastases. Most of the tumors (47.77%) were G3. Lymphatic invasion was noted in 18.68% of patients, whereas vascular invasion was detected in 15.07% of patients. Spread through air spaces was identified in 22.93% of patients. The most commonly occurring histological pattern was acinar (Table 5).
3.6. Survival Rates
Overall survival analysis is presented in Table 6 and Plot 1.
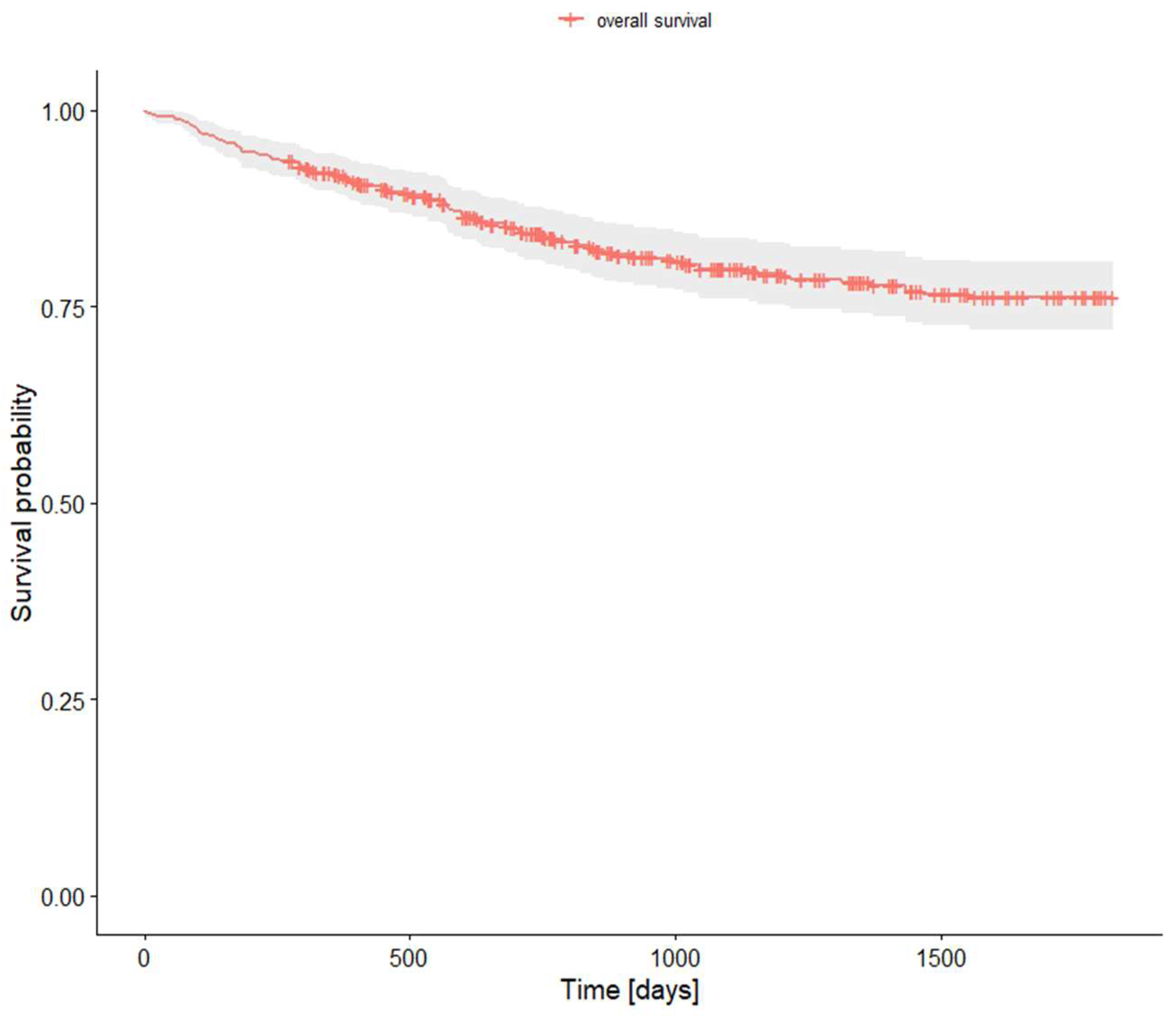
Plot 1. Survival analysis.
We found no differences in survival between G1 and G2 tumors, while both groups had significantly better survival rates compared to G3. Thus, we created a new variable that divides tumors into low grade (G1 and G2) and high grade (G3). Survival analysis for this division showed significant differences between low and high grade tumors. Survival rates for grading are presented in Table 7 and Plot 2.
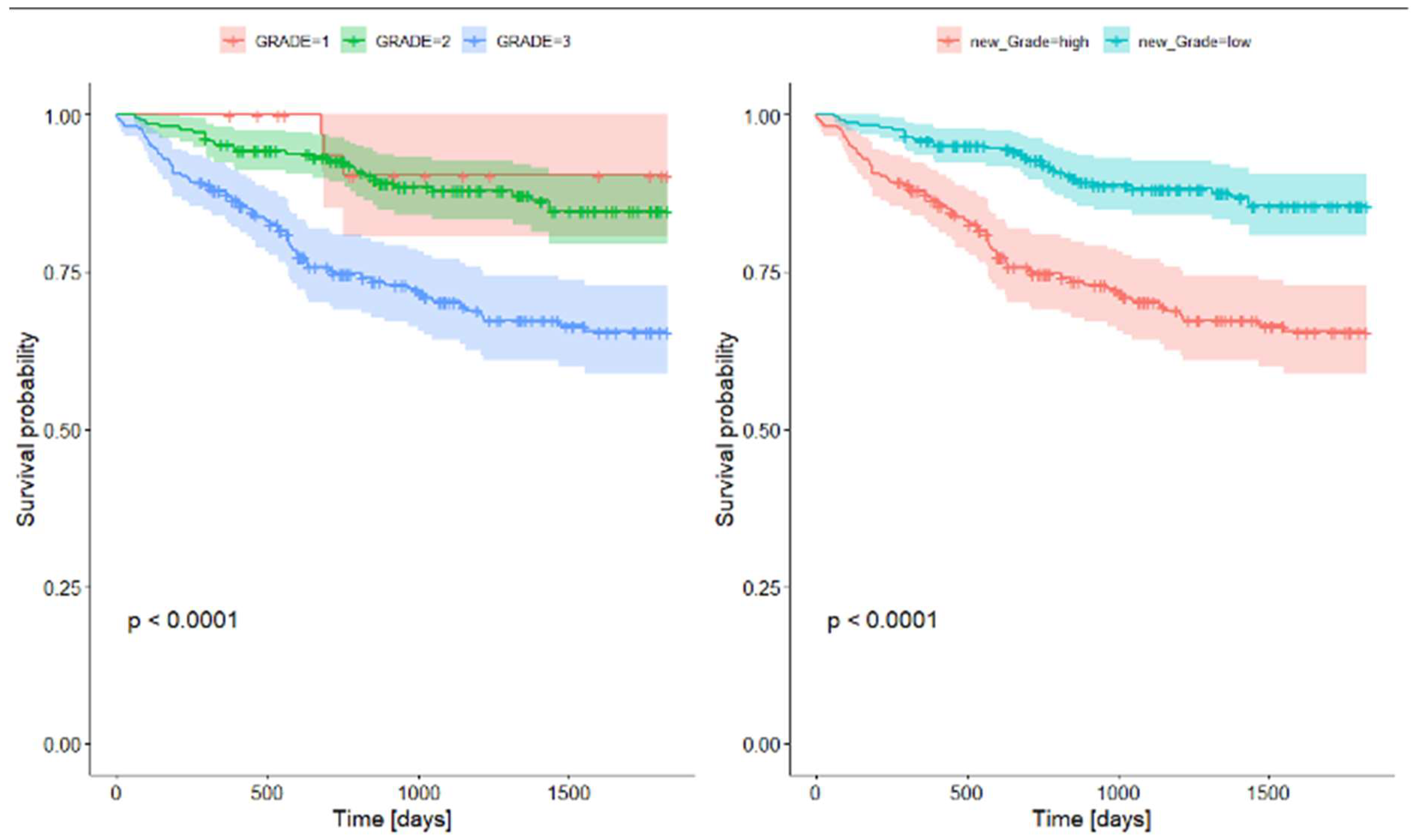
Plot 2. Survival rates for grading.
3.7. Survival Rates with STAS (Spread Through Air Spaces)
We found that there is a significant difference in survival rates between patients with STAS and those without it. Survival rates for STAS are presented in Table 8 and Plot 3.
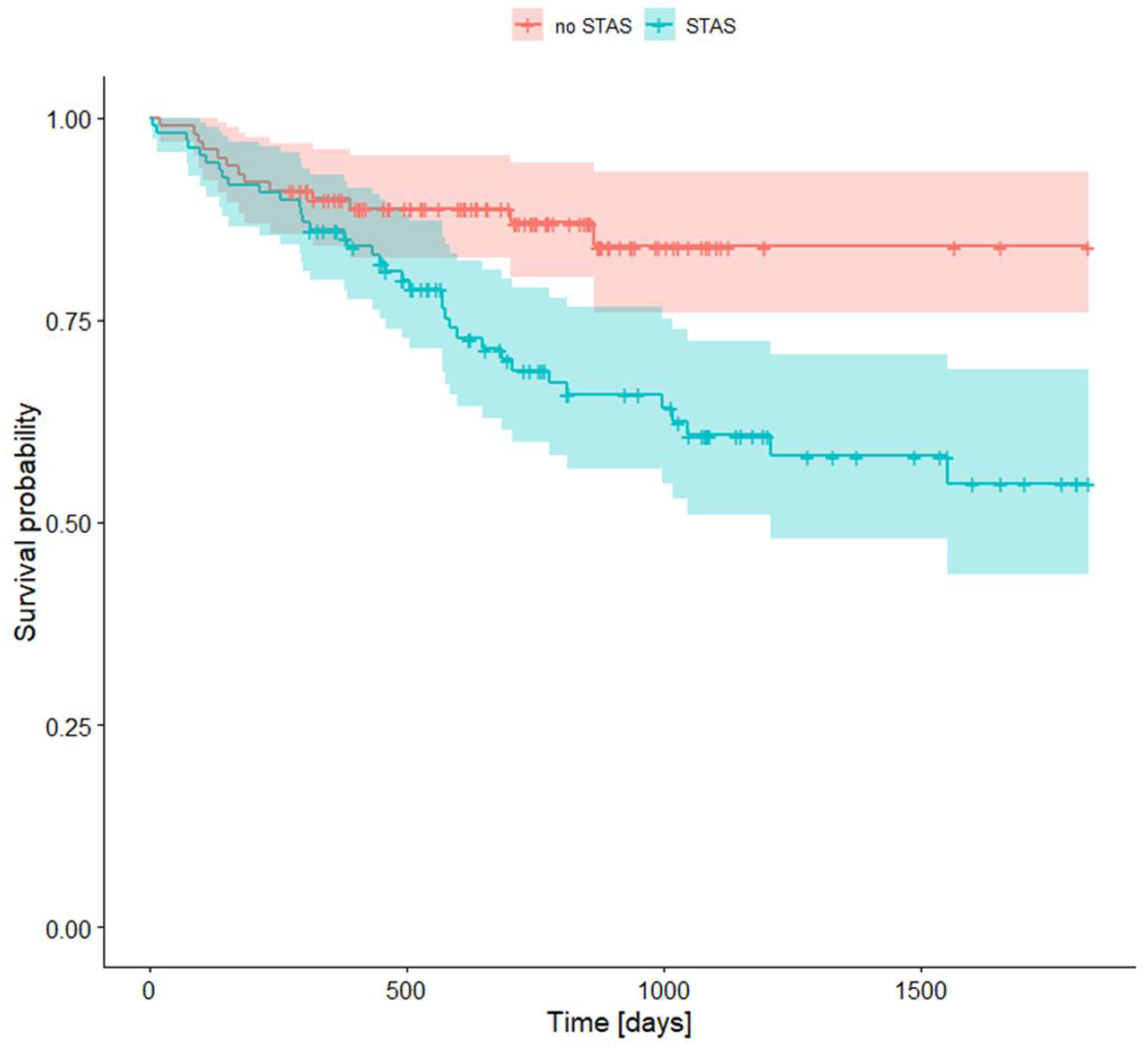
Plot 3. Survival rates for STAS presence.
4. Discussion
4.1. Symptoms
Due to the nonspecific nature of early clinical symptoms in lung carcinoma, most patients are symptomatic at the time of diagnosis [12]. According to the WHO, the most common symptoms are persistent cough, chest pain, shortness of breath, hemoptysis, fatigue, unexplained weight loss, and recurrent lung infections [9].
In our study, cough was the most common symptom of lung adenocarcinoma, occurring in 28.24% of patients. The remaining manifestations were pain (5.73%), hemoptysis (4.46%), and dyspnea (1.06%). The symptoms observed in our patient group are consistent with other studies [13,14,15].
According to Collins et al., primary tumor manifestations include chest discomfort, cough, dyspnea, and hemoptysis, aligning with our findings. Our cohort consisted of patients eligible for surgery, thus lacking symptoms linked to tumor metastasis. This factor contributes to the absence of symptoms associated with tumor spread in our group [12].
4.2. Staging
Clinical staging of NSCLC is an important factor that influences the choice of the most favorable treatment [16]. We revealed in our study that our patients were most commonly diagnosed with the early stage of lung adenocarcinoma. This group consisted of 416 patients (88.32%), of which 305 (64.75%) had stage I (n = 204 IA; n = 101 IB), and 111 (23.57%) had stage II (n = 38 IIA; n = 73 IIB). Similar results were also presented in a study by Fassi et al., in which the early stage of lung adenocarcinoma was diagnosed in 61% of patients, as well as in an article by Nasralla et al., which noted that 79.2% of NSCLC patients were diagnosed at stage I and 7.8% at stage II [16,17].
4.3. Grading
In line with the latest WHO tumor classification (5th edition), grade 1 tumors comprise lepidic-predominant tumors displaying less than 20% high-grade patterns. Acinar or papillary-predominant tumors featuring less than 20% high-grade patterns are designated as G2. Any tumor manifesting over 20% high-grade patterns (solid or micropapillary) falls under G3 classification [18]. In our study, we found no differences in overall survival between G1 and G2 tumor patients, while G3 tumors were characterized by significantly worse survival compared to the aforementioned G1 and G2. The two-tier system offers a simpler and more straightforward classification scheme, aiding clinicians in consistent interpretation and application. Furthermore, the two-tier system can reduce interobserver variability and improve interinstitutional agreement. By reducing the number of categories, discrepancies in grading across pathologists are minimized. Consequently, we advocate considering the simplification of the grading system. We found that segregating tumors based solely on the presence of 20% or more high-grade patterns enables the division of patients into two groups with notably distinct survival outcomes. Both grading systems retained significance even after adjusting for the stage variable. Moreira et al. suggested a three-tier grading system based on histologic patterns that was found to be a strong prognostic classifier [19].
4.4. Vascular and Pleural Invasion
Pleural and vascular invasion constitute the primary pathways through which lung adenocarcinoma spreads [20]. Pleural invasion stands out as an adverse prognostic factor among patients with lung adenocarcinoma. This phenomenon can be classified into distinct groups: PL0 (absence of pleural invasion), PL1 (invasion extending beyond the elastic layer of the visceral pleura but without exposure on the pleural surface), PL2 (tumor invasion of the pleural surface), and PL3 (tumor invasion of the parietal pleura) [21].
In our research, pleural invasion was present in 29.31% of cases (PL1 = 19.75%, PL2 = 7.86%, PL3 = 1.7%). Vascular invasion was observed in 15.07% of patients. Ito et al. reported percentages of pleural and vascular invasion as 9% and 12.7%, respectively [22]. Our findings diverged from the study by Usui et al., which indicated a vascular invasion rate of 45.1%[23]. In a study focused on stage I LUAD, vascular invasion was 26% [24]. Poleri et al. reported that 21% of patients exhibited visceral pleura invasion and 17% showed vascular invasion; however, their study included adenocarcinoma among other NSCLC types [25].
4.5. Spread through Air Spaces (STAS)
STAS is defined as the spread of tumor cells into the air spaces of the lung parenchyma beyond the tumor's boundary. This phenomenon was initially defined by the WHO in 2015 as a distinctive form of lung adenocarcinoma dissemination [26]. In our study, STAS was detected in 22.93% of patients. Similar results were presented in other studies. According to Huang et al., STAS was present in 28.2–37.3% of cases across all stages of lung adenocarcinoma [27]. Furthermore, Yin et al.'s research indicates that STAS can be found in 14.8 to 56.4% of lung adenocarcinomas [28]. Similar results were also presented in the work by Gu et al., indicating a prevalence of STAS in 15% to 50% of patients [20].
In both of these studies, STAS was proven to be associated with a worse survival rate compared to the group without this route of dissemination, which is consistent with our research. In our study, the difference between survival rates of patients with STAS and without it was 86.1% to 89.9% in 1-year survival, 68.8% to 87.0% in 2-year survival, and 54.8% to 85.1% in 5-year survival.
4.6. Histological Type
To properly grade and describe lung adenocarcinoma one must always search for different histological subtypes for usually there is more than one present. We revealed in our study that the most commonly found histological type of lung adenocarcinoma among our patients was acinar (77.33%). The remaining patterns were solid (46.22%), lepidic (28%), micropapillary (24%), and papillary (18%). The percentage of histological subtypes of adenocarcinoma shown in our study differed from those presented in other research; however, some studies overlap with our results, noting that acinar is the most common histologic type [29,30,31]. According to research by Bertoglio et al., lepidic was the most common type, accounting for 43%, while acinar comprised only 11.9% [32].
4.7. Survival Rates
There are many factors affecting survival rates among patients with lung adenocarcinoma. Researchers emphasize the importance of age at diagnosis, sex, TNM staging, tumor size, histological pattern, and factors related to treatment in determining survival rates [33,34]. Garinet et al. claim that staging is one of the most important factors in survival rates [35]. American research calculated the 5-year survival rate for stage IA tumors as 71% and for stage IB tumors as 57.6%[33]. Conversely, a study from Turkey demonstrated survival rates of 81.2%, 61.2%, 42.3%, 46.9%, and 37.1% for patients at stages IA, IB, IIA, IIB, and IIIA, respectively [36]. Nevertheless, the 5-year survival rate for our patients did not differentiate between individual tumor stages and was calculated at 76.2%. It is noteworthy that all our patients were surgical candidates, initially not exceeding stage IIIA, and the majority underwent radical (R0) surgeries, which could explain the relatively high survival outcomes. Similar results were presented in the Garinet et al. study, which reported a 5-year survival rate of 70% [35]. Yang et al. suggested a 5-year survival rate of 45-65% [34]. Conversely, Urer et al. reported an inferior 5-year survival rate of 52.3% [36].
4.8. Comorbidities
The most commonly reported comorbidities among our patients affected the cardiovascular system, with hypertension leading, affecting 54.99% of patients. Other cardiovascular diseases included coronary disease (18.47%), past myocardial infarction (7.86%), heart failure, chronic venous disease, and stroke. Similar to other studies on lung carcinoma, cardiovascular diseases also emerged as frequent comorbidities, with incidences ranging from 12.9% to 43% [37]. Analogous to lung carcinoma, the risk of developing most CVDs also increases with age [37]. It's noteworthy that approximately 60% of the population develops hypertension by the age of 60, and the mean age within our population is 65.9 years [38].
The second most prevalent group of comorbidities involved the respiratory system, with COPD standing out as the most frequently reported, aligning with existing data [37,39,40]. Both lung carcinoma and COPD share similar risk factors such as tobacco smoke exposure, air pollution, and older age. It is suggested that these two diseases are also closely linked at a molecular level, involving oxidative stress and inflammation [37,41].
Approximately 18% of patients from our database suffered from either insulin-dependent or non-insulin-dependent diabetes mellitus, which is comparable to the results of other studies [37,40,42].
Within our patient cohort, 15.9% reported a history of prior neoplastic diseases, with breast carcinoma being the most frequent, followed by prostate, urinary bladder, uterine, and other lung carcinomas. The prevalence of previous neoplasms corresponds with existing studies on lung carcinoma patients [40,43].
5. Conclusions
The symptoms of lung cancer were evident in over 60% of patients, with cough being the most frequently reported symptom at 28.24%. Notably, 88.32% of our patients were diagnosed at an early stage of lung adenocarcinoma. It was found that the presence of STAS (spread through air spaces) is associated with a worse survival rate compared to those without this route of spread. Among our patients, the acinar histological type of lung adenocarcinoma was the most common, accounting for 77.33%. The overall survival rate for patients with adenocarcinoma undergoing surgery was 76.23%. The lack of significant survival differences between G1 and G2 patients suggests that a two-tier grading system might be more appropriate than the current three-tier system. Additionally, the most prevalent comorbidities reported predominantly affected the cardiovascular system, followed by the respiratory system, with COPD being the most common respiratory comorbidity.
Author Contributions
P.Z., P. K..; Data curation, P.Z. N.Z., J.W., M.S., Z.S.; Formal analysis, P.Z., P.K.; Funding acquisition, P.Z. and B.D.; Investigation, P.Z., P.K. P.Z. N.Z., J.W., M.S., Z.S; Methodology, P.Z. and P.K.; Project administration, M.R., D.C. and B.D.; Resources, P.Z., M.R., M.B., D.C. and B.D.; Software, P.Z. and P.K.; Supervision, D.C. and B.D.; Validation, D.C. and B.D.; Writing—original draft, P.Z.; Writing—review & editing, P.Z. and B.D. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Institutional Review Board (or Ethics Committee) of Medical University of Silesia (No PCN/0022/KB/27/21).
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available in this article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Pan, B.; Wan, L.; Li, Y.; Yang, J.; Chen, Z.; Tong, X.; Zhao, J.; Li, C. Comprehensive Pan-Cancer Analysis Identifies FHL2 Associated with Poor Prognosis in Lung Adenocarcinoma. Translational Cancer Research 2023, 12. [Google Scholar] [CrossRef] [PubMed]
- Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries - Sung - 2021 - CA: A Cancer Journal for Clinicians - Wiley Online Library. Available online: https://acsjournals.onlinelibrary.wiley.com/doi/10.3322/caac.21660 (accessed on 17 September 2024).
- Min, S.; Zheng, Q. Clinicopathological and Prognostic Significance of NM23 Expression in Patients with Non-Small Cell Lung Cancer: A Systematic Review and Meta-Analysis. Medicine 2021, 100, e27919. [Google Scholar] [CrossRef] [PubMed]
- MALAT-1 Expression Correlates with Prognosis in Non-Small-Cell Lung Carcinoma: A Systematic Review and Meta-analysis - Ran - 2021 - Disease Markers - Wiley Online Library. Available online: https://onlinelibrary.wiley.com/doi/10.1155/2021/5424623 (accessed on 17 September 2024).
- Fan, K.; Zhang, C.; Qi, Y.; Dai, X.; Birling, Y.; Tan, Z.; Cao, F. Prognostic Value of EZH2 in Non-Small-Cell Lung Cancers: A Meta-Analysis and Bioinformatics Analysis. BioMed Research International 2020, 2020, 2380124. [Google Scholar] [CrossRef] [PubMed]
- Systematic Analyses of a Chemokine Family-Based Risk Model Predicting Clinical Outcome and Immunotherapy Response in Lung Adenocarcinoma - Jiarui Chen, Xingyu Liu, Qiuji Wu, Xueping Jiang, Zihang Zeng, Jiali Li, Yanping Gao, Yan Gong, Conghua Xie, 2021. Available online: https://journals.sagepub.com/doi/10.1177/09636897211055046 (accessed on 17 September 2024).
- Abdeahad, H.; Salehi, M.; Yaghoubi, A.; Aalami, A.H.; Aalami, F.; Soleimanpour, S. Previous Pulmonary Tuberculosis Enhances the Risk of Lung Cancer: Systematic Reviews and Meta-Analysis. Infectious Diseases 2022, 54, 255–268. [Google Scholar] [CrossRef] [PubMed]
- Screening Rate and Influential Factors of Lung Cancer with Low-Dose Computed Tomography in Asian Population: A Systematic Review and Meta-Analysis | Journal of Public Health | Oxford Academic. Available online: https://academic.oup.com/jpubhealth/article/44/2/246/6043299 (accessed on 17 September 2024).
- Lung Cancer. Available online: https://www.who.int/news-room/fact-sheets/detail/lung-cancer (accessed on 17 September 2024).
- Prognostic and Clinicopathological Significance of Long Noncoding RNA MALAT-1 Expression in Patients with Non-Small Cell Lung Cancer: A Meta-Analysis | PLOS ONE. Available online: https://journals.plos.org/plosone/article?id=10.1371/journal.pone.0240321 (accessed on 17 September 2024).
- Ochman, B.; Kiczmer, P.; Ziora, P.; Rydel, M.; Borowiecki, M.; Czyżewski, D.; Drozdzowska, B. Incidence of Concomitant Neoplastic Diseases, Tumor Characteristics, and the Survival of Patients with Lung Adenocarcinoma or Squamous Cell Lung Carcinoma in Tobacco Smokers and Non-Smokers—10-Year Retrospective Single-Centre Cohort Study. Cancers 2023, 15, 1896. [Google Scholar] [CrossRef] [PubMed]
- Collins, L.G.; Haines, C.; Perkel, R.; Enck, R.E. Lung Cancer: Diagnosis and Management. afp 2007, 75, 56–63. [Google Scholar]
- Lung Cancer - ClinicalKey. Available online: https://www.clinicalkey.com/#!/content/playContent/1-s2.0-S0025712518301718?returnurl=https:%2F%2Flinkinghub.elsevier.com%2Fretrieve%2Fpii%2FS0025712518301718%3Fshowall%3Dtrue&referrer=https:%2F%2Fpubmed.ncbi.nlm.nih.gov%2F (accessed on 17 September 2024).
- Kocher, F.; Hilbe, W.; Seeber, A.; Pircher, A.; Schmid, T.; Greil, R.; Auberger, J.; Nevinny-Stickel, M.; Sterlacci, W.; Tzankov, A.; et al. Longitudinal Analysis of 2293 NSCLC Patients: A Comprehensive Study from the TYROL Registry. Lung Cancer 2015, 87, 193–200. [Google Scholar] [CrossRef]
- Beckles, M.A.; Spiro, S.G.; Colice, G.L.; Rudd, R.M. Initial Evaluation of the Patient With Lung Cancer*: Symptoms, Signs, Laboratory Tests, and Paraneoplastic Syndromes. CHEST 2003, 123, 97S–104S. [Google Scholar] [CrossRef]
- Elevated Preoperative CEA Is Associated with Subclinical Nodal Involvement and Worse Survival in Stage I Non-Small Cell Lung Cancer: A Systematic Review and Meta-Analysis | Journal of Cardiothoracic Surgery | Full Text. Available online: https://cardiothoracicsurgery.biomedcentral.com/articles/10.1186/s13019-020-01353-2 (accessed on 17 September 2024).
- Fassi, E.; Mandruzzato, M.; Zamparini, M.; Bianchi, S.; Petrelli, F.; Baggi, A.; Alberti, A.; Grisanti, S.; Berruti, A. Clinical Presentation and Outcome of Patients with Enteric-Type Adenocarcinoma of the Lung: A Pooled Analysis of Published Cases. Lung Cancer 2023, 179. [Google Scholar] [CrossRef]
- Board, W.C. of T.E. Thoracic Tumours; ISBN 978-92-832-4506-3.
- A Grading System for Invasive Pulmonary Adenocarcinoma: A Proposal From the International Association for the Study of Lung Cancer Pathology Committee - Journal of Thoracic Oncology. Available online: https://www.jto.org/article/S1556-0864(20)30468-8/fulltext (accessed on 17 September 2024).
- Gu, Y.; Zheng, B.; Zhao, T.; Fan, Y. Computed Tomography Features and Tumor Spread Through Air Spaces in Lung Adenocarcinoma: A Meta-Analysis. Journal of Thoracic Imaging 2023, 38, W19. [Google Scholar] [CrossRef]
- Extent of Visceral Pleural Invasion and the Prognosis of Surgically Resected Node-negative Non-small Cell Lung Cancer - Seok - 2017 - Thoracic Cancer - Wiley Online Library. Available online: https://onlinelibrary.wiley.com/doi/10.1111/1759-7714.12424 (accessed on 17 September 2024).
- Second Predominant Subtype Predicts Outcomes of Intermediate-Malignant Invasive Lung Adenocarcinoma† | European Journal of Cardio-Thoracic Surgery | Oxford Academic. Available online: https://academic.oup.com/ejcts/article/51/2/218/2670152 (accessed on 17 September 2024).
- Usui, S.; Minami, Y.; Shiozawa, T.; Iyama, S.; Satomi, K.; Sakashita, S.; Sato, Y.; Noguchi, M. Differences in the Prognostic Implications of Vascular Invasion between Lung Adenocarcinoma and Squamous Cell Carcinoma. Lung Cancer 2013, 82, 407–412. [Google Scholar] [CrossRef] [PubMed]
- Yambayev, I.; Sullivan, T.B.; Rieger-Christ, K.M.; Servais, E.L.; Stock, C.T.; Quadri, S.M.; Sands, J.M.; Suzuki, K.; Burks, E.J. Vascular Invasion Identifies the Most Aggressive Histologic Subset of Stage I Lung Adenocarcinoma: Implications for Adjuvant Therapy. Lung Cancer 2022, 171, 82–89. [Google Scholar] [CrossRef] [PubMed]
- Poleri, C.; Morero, J.L.; Nieva, B.; Vaézquez, M.F.; Rodriéguez, C.; Titto, E. de; Rosenberg, M. Risk of Recurrence in Patients With Surgically Resected Stage I Non-Small Cell Lung Carcinomaa: Histopathologic and Immunohistochemical Analysis. CHEST 2003, 123, 1858–1867. [Google Scholar] [CrossRef] [PubMed]
- Travis, W.D.; Brambilla, E.; Nicholson, A.G.; Yatabe, Y.; Austin, J.H.M.; Beasley, M.B.; Chirieac, L.R.; Dacic, S.; Duhig, E.; Flieder, D.B.; et al. The 2015 World Health Organization Classification of Lung Tumors: Impact of Genetic, Clinical and Radiologic Advances Since the 2004 Classification. Journal of Thoracic Oncology 2015, 10, 1243–1260. [Google Scholar] [CrossRef] [PubMed]
- The Prognostic Significance of Tumor Spread through Air Space in Stage I Lung Adenocarcinoma - Huang - 2022 - Thoracic Cancer - Wiley Online Library. Available online: https://onlinelibrary.wiley.com/doi/10.1111/1759-7714.14348 (accessed on 17 September 2024).
- Yin, Q.; Wang, H.; Cui, H.; Wang, W.; Yang, G.; Qie, P.; Xun, X.; Han, S.; Liu, H. Meta-Analysis of Association between CT-Based Features and Tumor Spread through Air Spaces in Lung Adenocarcinoma. Journal of Cardiothoracic Surgery 2020, 15, 243. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Chung, Y.S.; Kim, K.A.; Shim, H.S. Prognostic Factors of Acinar- or Papillary-Predominant Adenocarcinoma of the Lung. Lung Cancer 2019, 137, 129–135. [Google Scholar] [CrossRef]
- Hung, J.-J.; Yeh, Y.-C.; Jeng, W.-J.; Wu, K.-J.; Huang, B.-S.; Wu, Y.-C.; Chou, T.-Y.; Hsu, W.-H. Predictive Value of the International Association for the Study of Lung Cancer/American Thoracic Society/European Respiratory Society Classification of Lung Adenocarcinoma in Tumor Recurrence and Patient Survival. JCO 2014, 32, 2357–2364. [Google Scholar] [CrossRef]
- Fujikawa, R.; Muraoka, Y.; Kashima, J.; Yoshida, Y.; Ito, K.; Watanabe, H.; Kusumoto, M.; Watanabe, S.; Yatabe, Y. Clinicopathologic and Genotypic Features of Lung Adenocarcinoma Characterized by the International Association for the Study of Lung Cancer Grading System. Journal of Thoracic Oncology 2022, 17, 700–707. [Google Scholar] [CrossRef]
- Prognostic Impact of Lung Adenocarcinoma Second Predominant Pattern from a Large European Database - Bertoglio - 2021 - Journal of Surgical Oncology - Wiley Online Library. Available online: https://onlinelibrary.wiley.com/doi/10.1002/jso.26292 (accessed on 17 September 2024).
- Prognostic Factors for Survival of Stage I Nonsmall Cell Lung Cancer Patients - Ou - 2007 - Cancer - Wiley Online Library. Available online: https://acsjournals.onlinelibrary.wiley.com/doi/10.1002/cncr.22938 (accessed on 17 September 2024).
- A Systematic Review and Meta-Analysis of the Influence of STAS on the Long-Term Prognosis of Stage I Lung Adenocarcinoma - Yang - Translational Cancer Research. Available online: https://tcr.amegroups.org/article/view/52391/html (accessed on 17 September 2024).
- Cancers | Free Full-Text | Updated Prognostic Factors in Localized NSCLC. Available online: https://www.mdpi.com/2072-6694/14/6/1400 (accessed on 17 September 2024).
- Relationship between Lung Adenocarcinoma Histological Subtype and Patient Prognosis. Available online: https://www.jstage.jst.go.jp/article/atcs/20/1/20_oa.12.02073/_article (accessed on 17 September 2024).
- Dutkowska, A.E.; Antczak, A. Comorbidities in Lung Cancer. Advances in Respiratory Medicine 2016, 84, 186–192. [Google Scholar] [CrossRef]
- Hypertension in Older Adults: Assessment, Management, and Challenges - Oliveros - 2020 - Clinical Cardiology - Wiley Online Library. Available online: https://onlinelibrary.wiley.com/doi/10.1002/clc.23303 (accessed on 17 September 2024).
- Tammemagi, C.M.; Neslund-Dudas, C.; Simoff, M.; Kvale, P. Smoking and Lung Cancer Survival: The Role of Comorbidity and Treatment. CHEST 2004, 125, 27–37. [Google Scholar] [CrossRef]
- Tammemagi, C.M.; Neslund-Dudas, C.; Simoff, M.; Kvale, P. In Lung Cancer Patients, Age, Race-Ethnicity, Gender and Smoking Predict Adverse Comorbidity, Which in Turn Predicts Treatment and Survival. Journal of Clinical Epidemiology 2004, 57, 597–609. [Google Scholar] [CrossRef] [PubMed]
- Durham, A.L.; Adcock, I.M. The Relationship between COPD and Lung Cancer. Lung Cancer 2015, 90, 121–127. [Google Scholar] [CrossRef] [PubMed]
- Islam, K.M.M.; Jiang, X.; Anggondowati, T.; Lin, G.; Ganti, A.K. Comorbidity and Survival in Lung Cancer Patients. Cancer Epidemiology, Biomarkers & Prevention 2015, 24, 1079–1085. [Google Scholar] [CrossRef]
- Aguiló, R.; Macià, F.; Porta, M.; Casamitjana, M.; Minguella, J.; Novoa, A.M. Multiple Independent Primary Cancers Do Not Adversely Affect Survival of the Lung Cancer Patient. European Journal of Cardio-Thoracic Surgery 2008, 34, 1075–1080. [Google Scholar] [CrossRef]
Table 1.
General characteristics of the study group.
| mean | SD | 95% CI | 95% CI | |
| Age | 65,9 | 7,81 | 65.19 | 66.61 |
| Gender | n | % | 95% CI | 95% CI |
| Female | 252,00 | 53,50% | 49,00% | 58,01% |
| Male | 219,00 | 46,50% | 41,99% | 51,00% |
| Smoking Status | n | % | 95% CI | 95% CI |
| Smoker | 356 | 75,58% | 71,70% | 79,46% |
| Non-smoker | 115 | 24,42% | 20,54% | 28,30% |
Table 2.
Former cancer history among patients.
| Former neoplastic diseases | n | % | 95% CI | 95% CI |
|---|---|---|---|---|
| Any former neoplastic diseases | 75 | 15,89% | 12,59% | 19,19% |
| Breast | 16 | 3,39% | 1,76% | 5,02% |
| Prostate | 12 | 2,54% | 1,12% | 3,96% |
| Urinary bladder | 10 | 2,12% | 0,82% | 3,42% |
| Uterus | 7 | 1,48% | 0,39% | 2,57% |
| Lung | 7 | 1,48% | 0,39% | 2,57% |
| Large intestine | 5 | 1,06% | 0,13% | 1,98% |
| Kidney | 4 | 0,85% | 0,02% | 1,68% |
| Larynx | 2 | 0,42% | 0,00% | 1,01% |
| Ovary | 2 | 0,42% | 0,00% | 1,01% |
| Stomach | 2 | 0,42% | 0,00% | 1,01% |
| Other | 12 | 2,54% | 1,12% | 3,96% |
| Neoplasms in family | 102 | 21,61% | 17,89% | 25,33% |
Table 3.
Comorbidities among patients.
| Comorbidities | n | % | 95% CI | 95% CI |
|---|---|---|---|---|
| Insulin-dependent diabetes mellitus, IDDM | 4,00 | 0,85% | 0,02% | 1,68% |
| Non insulin-dependent diabetes mellitus, NIDDM | 80,00 | 16,99% | 13,59% | 20,38% |
| Myocardial infarction in the past | 37,00 | 7,86% | 5,43% | 10,29% |
| Heart failure | 7,00 | 1,49% | 0,39% | 2,58% |
| Renal failure | 3,00 | 0,64% | 0,00% | 1,36% |
| COPD | 73,00 | 15,50% | 12,23% | 18,77% |
| Bronchial asthma | 22,00 | 4,67% | 2,77% | 6,58% |
| Epilepsy | 0,00 | 0,00% | 0,00% | 0,00% |
| Stroke in the past | 1,00 | 0,21% | 0,00% | 0,63% |
| Hypertension | 259,00 | 54,99% | 50,50% | 59,48% |
| Coronary disease | 87,00 | 18,47% | 14,97% | 21,98% |
| Blood coagulation disorders | 1,00 | 0,21% | -0,20% | 0,63% |
| Chronic venous disease | 7,00 | 1,49% | 0,39% | 2,58% |
Table 4.
Cancer symptoms among patients.
| Cancer symptoms | n | % | 95% CI | 95% CI |
|---|---|---|---|---|
| Any cancer symptoms | 284,00 | 60,30% | 55,88% | 64,72% |
| Cough | 133 | 28,24% | 24,17% | 32,30% |
| Pain | 27,00 | 5,73% | 3,63% | 7,83% |
| Haemoptysis | 21,00 | 4,46% | 2,59% | 6,32% |
| Dyspnoea | 5,00 | 1,06% | 0,14% | 1,99% |
Table 5.
Tumor characteristics.
| n | % | 95% CI | 95% CI | |
|---|---|---|---|---|
| pT parameter | ||||
| pT1a | 17 | 3,61% | 1,92% | 5,29% |
| pT1b | 107 | 22,72% | 18,93% | 26,50% |
| pT1c | 105 | 22,29% | 18,53% | 26,05% |
| pT2a | 118 | 25,05% | 21,14% | 28,97% |
| pT2b | 45 | 9,55% | 6,90% | 12,21% |
| pT3 | 51 | 10,83% | 8,02% | 13,63% |
| pT4 | 28 | 5,94% | 3,81% | 8,08% |
| pN parameter | ||||
| pN0 | 399 | 84,71% | 81,46% | 87,96% |
| pN1 | 41 | 8,70% | 6,16% | 11,25% |
| pN2 | 31 | 6,58% | 4,34% | 8,82% |
| pM parameter | ||||
| pM0 | 471 | 100,00% | 100,00% | 100,00% |
| Stage | ||||
| IA1 | 17 | 3,61% | 1,92% | 5,29% |
| IA2 | 97 | 20,59% | 16,94% | 24,25% |
| IA3 | 90 | 19,11% | 15,56% | 22,66% |
| IB | 101 | 21,44% | 17,74% | 25,15% |
| IIA | 38 | 8,07% | 5,61% | 10,53% |
| IIB | 73 | 15,50% | 12,23% | 18,77% |
| IIIA | 38 | 8,07% | 5,61% | 10,53% |
| IIIB | 16 | 3,40% | 1,76% | 5,03% |
| IVA | 1 | 0,21% | 0,00% | 0,63% |
| Grading | ||||
| G1 | 35 | 7,43% | 5,06% | 9,80% |
| G2 | 211 | 44,80% | 40,31% | 49,29% |
| G3 | 225 | 47,77% | 43,26% | 52,28% |
| Vascular invasion | ||||
| Lymphatic invasion | 88 | 18,68% | 15,16% | 22,20% |
| Vascular invasion | 71 | 15,07% | 11,84% | 18,31% |
| Pleural invasion | ||||
| 0 | 330 | 70,06% | 65,93% | 74,20% |
| 1 | 93 | 19,75% | 16,15% | 23,34% |
| 2 | 37 | 7,86% | 5,43% | 10,29% |
| 3 | 8 | 1,70% | 0,53% | 2,87% |
| Radical resection | ||||
| R0 | 439 | 93,21% | 90,93% | 95,48% |
| R1 | 18 | 3,82% | 2,09% | 5,55% |
| Spread through air spaces | ||||
| STAS | 108 | 22,93% | 19,13% | 26,73% |
| Histologic pattern | ||||
| n | % | 95% CI | 95% CI | |
| lepidic | 126 | 28% | 23,85% | 32,15% |
| acinar | 348 | 77,33% | 73,46% | 81,20% |
| papillary | 81 | 18% | 14,45% | 21,55% |
| micropapillary | 108 | 24% | 20,05% | 27,95% |
| solid | 208 | 46,22% | 41,61% | 50,83% |
Table 6.
Survival analysis.
| Research population | |||
|---|---|---|---|
| Survival Rate | 95% CI | ||
| 1-year survival rate | 0,919000 | 0,895 | 0,944 |
| 2-years survival rate | 0,843277 | 0,81 | 0,878 |
| 5-years survival rate | 0,762390 | 0,721 | 0,806 |
Table 7.
Survival rates for grading.
| Low grade | High grade | |||||
|---|---|---|---|---|---|---|
| Survival Rate | 95% CI | Survival Rate | 95% CI | |||
| 1-year survival rate | 0,959 | 0,935 | 0,984 | 0,875 | 0,833 | 0,919 |
| 2-years survival rate | 0,928 | 0,896 | 0,962 | 0,747 | 0,69 | 0,809 |
| 5-years survival rate | 0,865 | 0,808 | 0,906 | 0,655 | 0,588 | 0,729 |
Table 8.
Survival rates for STAS presence.
| STAS + | STAS - | |||||
|---|---|---|---|---|---|---|
| Survival Rate | 95% CI | Survival Rate | 95% CI | |||
| 1-year survival rate | 0,861 | 0,798 | 0,929 | 0,899 | 0,842 | 0,961 |
| 2-years survival rate | 0,688 | 0,599 | 0,790 | 0,870 | 0,803 | 0,943 |
| 5-years survival rate | 0,548 | 0,436 | 0,688 | 0,841 | 0,758 | 0,934 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Copyright: This open access article is published under a Creative Commons CC BY 4.0 license, which permit the free download, distribution, and reuse, provided that the author and preprint are cited in any reuse.
A Single Center Experience in Combined Oncological-Surgical Treatment for Resectable Locally-Advanced Non-small Cell Lung Cancer (NSCLC)
Dan Levy Faber
et al.
,
2024
Epidemiologic and Histopathologic Feature of Lung Cancer in Central Iran (2012-2018)
Mohammad Sadegh Khalilian
et al.
,
2020
MDPI Initiatives
Important Links
© 2024 MDPI (Basel, Switzerland) unless otherwise stated








