Neurologic manifestation of HIV/AIDS
- 1. NEUROLOGIC MANIFESTATION OF HIV/AIDS (INDIA) Dr PS Deb, MD, DM
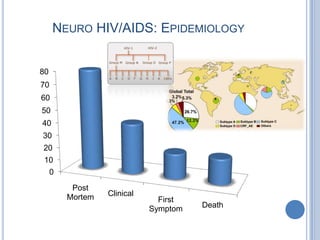
- 2. NEURO HIV/AIDS: EPIDEMIOLOGY 80 70 60 50 40 30 20 10 0 Post Mortem Clinical First Symptom Death
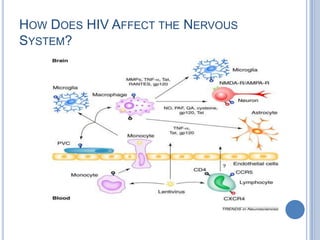
- 3. HOW DOES HIV AFFECT THE NERVOUS SYSTEM?
- 4. NEURO HIV/AIDS CLASSIFICATION: ICD10 B21: Neoplasm B20: Oppor. Infection • (B21.0) HIV disease resulting in Kaposi's sarcom • (B21.2) HIV disease resulting in other types of non-Hodgkin's lymphoma • (B20.0) HIV disease resulting in mycobacterial infection • (B21.3) HIV disease resulting in • (B20.1) HIV disease resulting in other malignant neoplasms other bacterial infections of lymphoid, haematopoietic and related • (B20.2) HIV disease resulting tissue in cytomegaloviral disease • (B21.7) HIV disease resulting in • (B20.3) HIV disease resulting in other viral multiple malignant neoplasms infections • (B21.8) HIV disease resulting in • (B20.8) HIV disease resulting in other malignant neoplasms other infectious and parasitic diseases • (B21.9) HIV disease resulting in • (B20.9) HIV disease resulting in unspecified malignant neoplasm unspecified infectious or parasitic disease B22: Specific Syndromes • (B22.0) HIV disease resulting B23: Other in encephalopathy B24: NOS
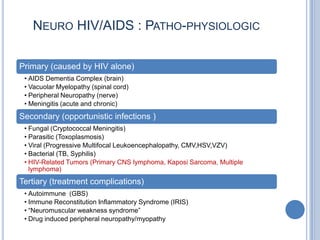
- 5. NEURO HIV/AIDS : PATHO-PHYSIOLOGIC Primary (caused by HIV alone) • AIDS Dementia Complex (brain) • Vacuolar Myelopathy (spinal cord) • Peripheral Neuropathy (nerve) • Meningitis (acute and chronic) Secondary (opportunistic infections ) • Fungal (Cryptococcal Meningitis) • Parasitic (Toxoplasmosis) • Viral (Progressive Multifocal Leukoencephalopathy, CMV,HSV,VZV) • Bacterial (TB, Syphilis) • HIV-Related Tumors (Primary CNS lymphoma, Kaposi Sarcoma, Multiple lymphoma) Tertiary (treatment complications) • Autoimmune (GBS) • Immune Reconstitution Inflammatory Syndrome (IRIS) • “Neuromuscular weakness syndrome” • Drug induced peripheral neuropathy/myopathy
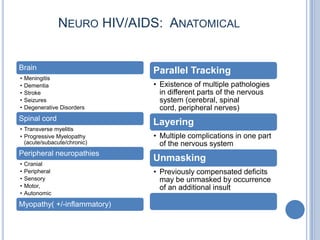
- 6. NEURO HIV/AIDS: ANATOMICAL Brain Parallel Tracking • Meningitis • Dementia • Existence of multiple pathologies • Stroke in different parts of the nervous • Seizures system (cerebral, spinal • Degenerative Disorders cord, peripheral nerves) Spinal cord Layering • Transverse myelitis • Progressive Myelopathy • Multiple complications in one part (acute/subacute/chronic) of the nervous system Peripheral neuropathies Unmasking • Cranial • Peripheral • Previously compensated deficits • Sensory may be unmasked by occurrence • Motor, of an additional insult • Autonomic Myopathy( +/-inflammatory)
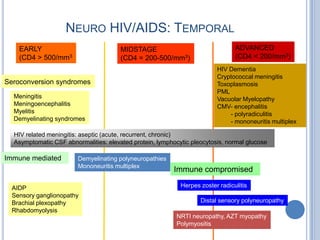
- 7. NEURO HIV/AIDS: TEMPORAL EARLY MIDSTAGE ADVANCED (CD4 > 500/mm3 (CD4 = 200-500/mm3) (CD4 < 200/mm3) HIV Dementia Cryptococcal meningitis Seroconversion syndromes Toxoplasmosis PML Meningitis Vacuolar Myelopathy Meningoencephalitis CMV- encephalitis Myelitis - polyradiculitis Demyelinating syndromes - mononeuritis multiplex HIV related meningitis: aseptic (acute, recurrent, chronic) Asymptomatic CSF abnormalities: elevated protein, lymphocytic pleocytosis, normal glucose Immune mediated Demyelinating polyneuropathies Mononeuritis multiplex Immune compromised AIDP Herpes zoster radiculitis Sensory ganglionopathy Brachial plexopathy Distal sensory polyneuropathy Rhabdomyolysis NRTI neuropathy, AZT myopathy Polymyositis
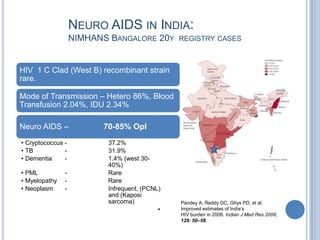
- 8. NEURO AIDS IN INDIA: NIMHANS BANGALORE 20Y REGISTRY CASES HIV 1 C Clad (West B) recombinant strain rare. Mode of Transmission – Hetero 86%, Blood Transfusion 2.04%, IDU 2.34% Neuro AIDS – 70-85% OpI • Cryptococcus - 37.2% • TB - 31.9% • Dementia - 1.4% (west 30- 40%) • PML - Rare • Myelopathy - Rare • Neoplasm - Infrequent, (PCNL) and (Kaposi sarcoma) Pandey A, Reddy DC, Ghys PD, et al. • Improved estimates of India’s HIV burden in 2006. Indian J Med Res 2009; 129: 50–58.
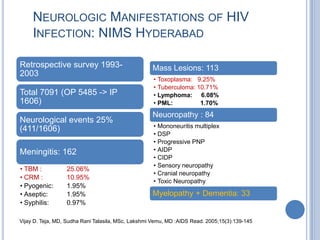
- 9. NEUROLOGIC MANIFESTATIONS OF HIV INFECTION: NIMS HYDERABAD Retrospective survey 1993- Mass Lesions: 113 2003 • Toxoplasma: 9.25% • Tuberculoma: 10.71% Total 7091 (OP 5485 -> IP • Lymphoma: 6.08% 1606) • PML: 1.70% Neuoropathy : 84 Neurological events 25% • Mononeuritis multiplex (411/1606) • DSP • Progressive PNP Meningitis: 162 • AIDP • CIDP • Sensory neuropathy • TBM : 25.06% • Cranial neuropathy • CRM : 10.95% • Toxic Neuropathy • Pyogenic: 1.95% • Aseptic: 1.95% Myelopathy + Dementia: 33 • Syphilis: 0.97% Vijay D. Teja, MD, Sudha Rani Talasila, MSc, Lakshmi Vemu, MD :AIDS Read. 2005;15(3):139-145
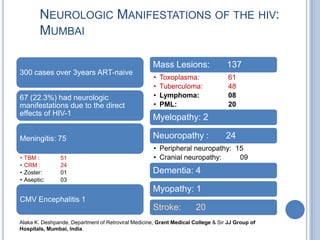
- 10. NEUROLOGIC MANIFESTATIONS OF THE HIV: MUMBAI Mass Lesions: 137 300 cases over 3years ART-naive • Toxoplasma: 61 • Tuberculoma: 48 67 (22.3%) had neurologic • Lymphoma: 08 manifestations due to the direct • PML: 20 effects of HIV-1 Myelopathy: 2 Meningitis: 75 Neuoropathy : 24 • Peripheral neuropathy: 15 • TBM : 51 • Cranial neuropathy: 09 • CRM : 24 • Zoster: 01 Dementia: 4 • Aseptic: 03 Myopathy: 1 CMV Encephalitis 1 Stroke: 20 Alaka K. Deshpande, Department of Retroviral Medicine, Grant Medical College & Sir JJ Group of Hospitals, Mumbai, India.
- 11. ASEPTIC MENINGITIS May occur in acute infection or sero-conversion or in the chronic stage of HIV infection Clinical • Fever, malaise, stiff neck, and photophobia • Clinical course is self-limited, without sequelae • Cranial neuropathy, typically Bell’s palsy, may co-exist • After recovery, underlying HIV may be asymptomatic Laboratory evaluation • CSF: lymphocytic pleocytosis; normal glucose and normal or slightly elevated protein • HIV serology: may be negative; repeat at 3 and 6 months • HIV antigen and viral determination positive • T cell studies: normal or borderline • EEG, CT or MRI of brain normal or non-diagnostic
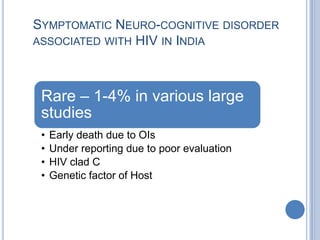
- 12. SYMPTOMATIC NEURO-COGNITIVE DISORDER ASSOCIATED WITH HIV IN INDIA Rare – 1-4% in various large studies • Early death due to OIs • Under reporting due to poor evaluation • HIV clad C • Genetic factor of Host
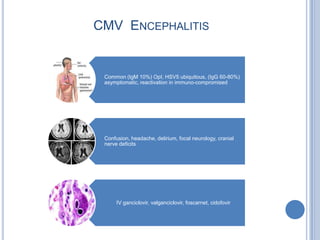
- 13. CMV ENCEPHALITIS Common (IgM 10%) OpI, HSV5 ubiquitous, (IgG 60-80%) asymptomatic, reactivation in immuno-compromised Confusion, headache, delirium, focal neurology, cranial nerve deficits IV ganciclovir, valganciclovir, foscarnet, cidofovir
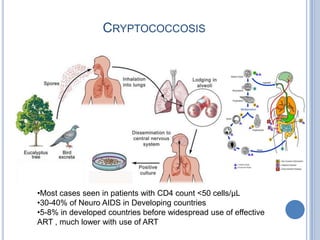
- 14. CRYPTOCOCCOSIS •Most cases seen in patients with CD4 count <50 cells/µL •30-40% of Neuro AIDS in Developing countries •5-8% in developed countries before widespread use of effective ART , much lower with use of ART
- 15. CRYPTOCOCCAL MENINGITIS • Fever, malaise, headache Subacute • Neck stiffness, photophobia, or other classic meningeal signs and symptoms in or Chronic 25-35% of cases • Lethargy, altered mental status, personality meningitis changes (less common) • Lymphocytic with raised protein, low sugar • India Ink CSF • Cr Ag in CSF • Cr culture in Blood
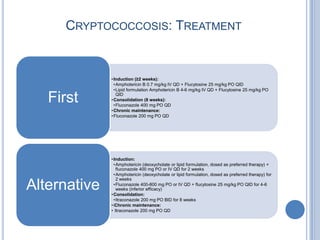
- 16. CRYPTOCOCCOSIS: TREATMENT • Induction (≥2 weeks): •Amphotericin B 0.7 mg/kg IV QD + Flucytosine 25 mg/kg PO QID •Lipid formulation Amphotericin B 4-6 mg/kg IV QD + Flucytosine 25 mg/kg PO First QID • Consolidation (8 weeks): •Fluconazole 400 mg PO QD • Chronic maintenance: • Fluconazole 200 mg PO QD • Induction: •Amphotericin (deoxycholate or lipid formulation, dosed as preferred therapy) + fluconazole 400 mg PO or IV QD for 2 weeks •Amphotericin (deoxycholate or lipid formulation, dosed as preferred therapy) for 2 weeks Alternative •Fluconazole 400-800 mg PO or IV QD + flucytosine 25 mg/kg PO QID for 4-6 weeks (inferior efficacy) • Consolidation: •Itraconazole 200 mg PO BID for 8 weeks • iChronic maintenance: • Itraconazole 200 mg PO QD
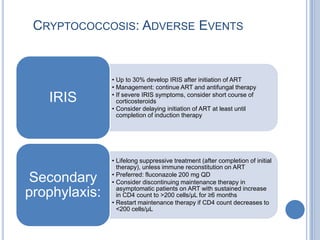
- 17. CRYPTOCOCCOSIS: ADVERSE EVENTS • Up to 30% develop IRIS after initiation of ART • Management: continue ART and antifungal therapy • If severe IRIS symptoms, consider short course of IRIS corticosteroids • Consider delaying initiation of ART at least until completion of induction therapy • Lifelong suppressive treatment (after completion of initial therapy), unless immune reconstitution on ART • Preferred: fluconazole 200 mg QD Secondary • Consider discontinuing maintenance therapy in asymptomatic patients on ART with sustained increase prophylaxis: in CD4 count to >200 cells/µL for ≥6 months • Restart maintenance therapy if CD4 count decreases to <200 cells/µL
- 18. TUBERCULOSIS IN HIV •WHO 2006 9.2millioon TB world and 7.7% were HIV + •TB in normal population 5-10% / 5- 15 % in HIV + •Role of HIV epidemic on TB epidemic in India is not clear
- 19. CLINICAL AND IMMUNO PATHOLOGICAL COURSE OF HIV ASSOCIATED TUBERCULOSIS (DE COCK ET AL 1992).
- 20. HIV AND NS TUBERCULOSIS 1/3 of all AIDS related deaths due to Tb. Untreated universally fatal, with low CD4 • HIV accelerates the spread of TB and latent to active by 100 fold • Accelerates the course of HIV so needs index of suspicion. • HAART has reduced TB by 80% in Brazil • India Human Mycobacterium is common not avian Clinical picture of TBM with/without HIV not much difference • Fever less common • Seizure more common • Hydrocephalus more common • Cerebral infarction more common • No difference in CSF picture • No difference in the response to the treatment
- 21. TREATMENT OF TB MENINGITIS Anti TB (HRZ+E/STM)x2 + (HR)x10 months (12-18months) Steroid: can be used as in non HIV case • Reduce cerebral edema, ICH, inflammation • Prevent hydrocephalus, vasculitis • Mortality benefit (41->31%) in Vietnam study
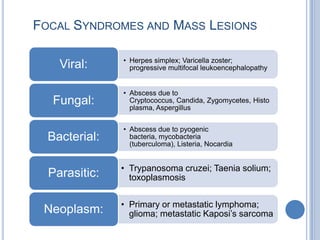
- 22. FOCAL SYNDROMES AND MASS LESIONS • Herpes simplex; Varicella zoster; Viral: progressive multifocal leukoencephalopathy • Abscess due to Fungal: Cryptococcus, Candida, Zygomycetes, Histo plasma, Aspergillus • Abscess due to pyogenic Bacterial: bacteria, mycobacteria (tuberculoma), Listeria, Nocardia • Trypanosoma cruzei; Taenia solium; Parasitic: toxoplasmosis • Primary or metastatic lymphoma; Neoplasm: glioma; metastatic Kaposi’s sarcoma
- 23. TOXOPLASMOSIS
- 24. TOXOPLASMOSIS
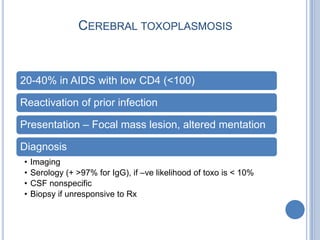
- 25. CEREBRAL TOXOPLASMOSIS 20-40% in AIDS with low CD4 (<100) Reactivation of prior infection Presentation – Focal mass lesion, altered mentation Diagnosis • Imaging • Serology (+ >97% for IgG), if –ve likelihood of toxo is < 10% • CSF nonspecific • Biopsy if unresponsive to Rx
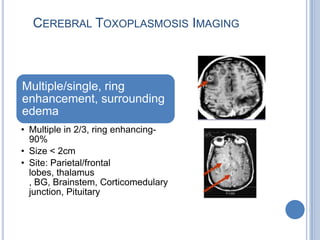
- 26. CEREBRAL TOXOPLASMOSIS IMAGING Multiple/single, ring enhancement, surrounding edema • Multiple in 2/3, ring enhancing- 90% • Size < 2cm • Site: Parietal/frontal lobes, thalamus , BG, Brainstem, Corticomedulary junction, Pituitary
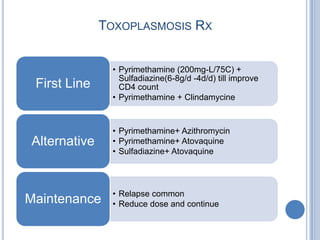
- 27. TOXOPLASMOSIS RX • Pyrimethamine (200mg-L/75C) + Sulfadiazine(6-8g/d -4d/d) till improve First Line CD4 count • Pyrimethamine + Clindamycine • Pyrimethamine+ Azithromycin Alternative • Pyrimethamine+ Atovaquine • Sulfadiazine+ Atovaquine • Relapse common Maintenance • Reduce dose and continue
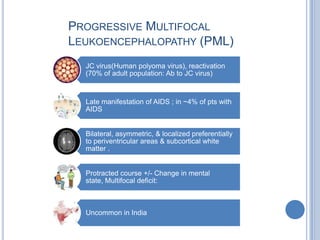
- 28. PROGRESSIVE MULTIFOCAL LEUKOENCEPHALOPATHY (PML) JC virus(Human polyoma virus), reactivation (70% of adult population: Ab to JC virus) Late manifestation of AIDS ; in ~4% of pts with AIDS Bilateral, asymmetric, & localized preferentially to periventricular areas & subcortical white matter . Protracted course +/- Change in mental state, Multifocal deficit: Uncommon in India
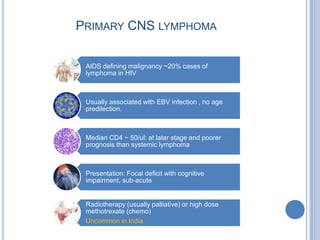
- 29. PRIMARY CNS LYMPHOMA AIDS defining malignancy ~20% cases of lymphoma in HIV Usually associated with EBV infection , no age predilection. Median CD4 ~ 50/ul: at later stage and poorer prognosis than systemic lymphoma Presentation: Focal deficit with cognitive impairment, sub-acute Radiotherapy (usually palliative) or high dose methotrexate (chemo) Uncommon in India
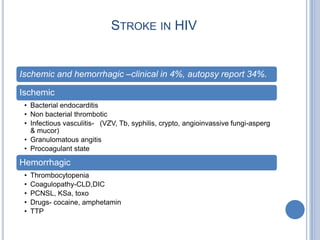
- 30. STROKE IN HIV Ischemic and hemorrhagic –clinical in 4%, autopsy report 34%. Ischemic • Bacterial endocarditis • Non bacterial thrombotic • Infectious vasculitis- (VZV, Tb, syphilis, crypto, angioinvassive fungi-asperg & mucor) • Granulomatous angitis • Procoagulant state Hemorrhagic • Thrombocytopenia • Coagulopathy-CLD,DIC • PCNSL, KSa, toxo • Drugs- cocaine, amphetamin • TTP
- 31. VACUOLAR MYELOPATHY Vacuolar Myelopathy • - 1/3 (20-55%) in autopsy series Clinical manifestation is much smaller • Usually late HIV • Develops slowly (months) • Coexisting neuropathy • Sensory symptoms: loss vibration and joint position sensation with relatively preserve pain sensation. • No discrete sensory level
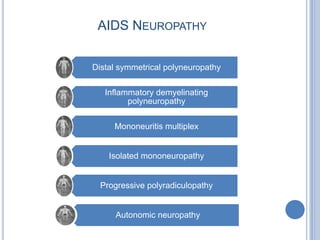
- 32. AIDS NEUROPATHY Distal symmetrical polyneuropathy Inflammatory demyelinating polyneuropathy Mononeuritis multiplex Isolated mononeuropathy Progressive polyradiculopathy Autonomic neuropathy
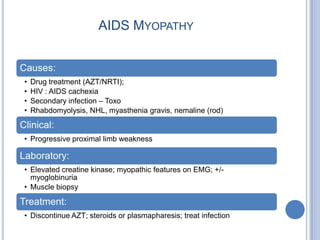
- 33. AIDS MYOPATHY Causes: • Drug treatment (AZT/NRTI); • HIV : AIDS cachexia • Secondary infection – Toxo • Rhabdomyolysis, NHL, myasthenia gravis, nemaline (rod) Clinical: • Progressive proximal limb weakness Laboratory: • Elevated creatine kinase; myopathic features on EMG; +/- myoglobinuria • Muscle biopsy Treatment: • Discontinue AZT; steroids or plasmapharesis; treat infection
Editor's Notes
- 10-15% of AIDS patients present with neurologic symptoms only (5% with dementia).35-50% of AIDS patients have neurologic symptoms during life1 (35% develop minor cognitive/motor disorder; 15-20% progress to dementia2)75-90% have neuropathologic abnormalities at death3 1) Brouwman et al, Neurology. 1998 ; 50:1814-20. 2) McArthur J Neuroimmunol 2004; 157 : 3-10 3) Vago et al., AIDS. 2002;16:1925-8.
- HIV can easily cross the blood brain barrier at early stage of infectionDo not affect directly CNS neurons or oligodendrocytesTheories HIV crossing BBBInfected monocytes and lymphocytes traffic across the BBB as part of their normal immune surveillance roleBlood brain barrier weakened by this process – leading to increased traffickingMonocytes differentiate in to microglia and macrophagesMeningeal macrophages infiltrate the CNS through the CSF compartmentA combination of these processesNeurotoxic viral proteins released in to CNS by HIV infected cells resulting in neuronal injury / death
- (B20) Human Immunodeficiency Virus (HIV) disease Resulting in infectious and parasitic diseases(B20.0) HIV disease resulting in mycobacterial infection(B20.1) HIV disease resulting in other bacterial infections(B20.2) HIV disease resulting in cytomegaloviral disease(B20.3) HIV disease resulting in other viral infections(B20.4) HIV disease resulting in candidiasis(B20.5) HIV disease resulting in other mycoses(B20.6) HIV disease resulting in Pneumocystis pneumonia(B20.7) HIV disease resulting in multiple infections(B20.8) HIV disease resulting in other infectious and parasitic diseases(B20.9) HIV disease resulting in unspecified infectious or parasitic disease(B21) Human Immunodeficiency Virus (HIV) disease Resulting in malignant neoplasms(B21.0) HIV disease resulting in Kaposi's sarcoma(B21.1) HIV disease resulting in Burkitt's lymphoma(B21.2) HIV disease resulting in other types of non-Hodgkin's lymphoma(B21.3) HIV disease resulting in other malignant neoplasms of lymphoid, haematopoietic and related tissue(B21.7) HIV disease resulting in multiple malignant neoplasms(B21.8) HIV disease resulting in other malignant neoplasms(B21.9) HIV disease resulting in unspecified malignant neoplasm(B22) Human Immunodeficiency Virus (HIV) disease Resulting in other specified diseases(B22.0) HIV disease resulting in encephalopathy(B22.1) HIV disease resulting in lymphoid interstitial pneumonitis(B22.2) HIV disease resulting in wasting syndrome(B22.7) HIV disease resulting in multiple diseases classified elsewhere(B23) Human Immunodeficiency Virus (HIV) disease Resulting in other conditions(B23.0) Acute HIV infection syndrome(B23.1) HIV disease resulting in (persistent) generalized lymphadenopathy(B23.2) HIV disease resulting in haematological and immunological abnormalities, not elsewhere classified(B23.8) HIV disease resulting in other specified conditions(B24) Unspecified Human Immunodeficiency Virus (HIV) Disease
- Kaposi Sarcoma In IndiaYear : 2010 | Volume : 76 | Issue : 2 | Page : 215Kaposi's sarcoma: A presenting sign of HIVJignesh B Vaishnani, Sanjay S Bosamiya, Anjum M MominSurat Municipal Institute of Medical, Education & Research (SMIMER), Umarwada, Surat - 395 009, Gujarat, IndiaKS was initially described by the Hungarian dermatologist, Morris Kaposi in 1872. There are four recognized clinical subsets of KS- Classical, Endemic (African), KS associated with non-HIV induced immunosuppression and with HIV infection (epidemic). HIV- associated KS was first recognized in 1979 when an epidemic of KS was identified in the homosexual community in New York. [1] The World Health Organization (WHO) clinical staging for HIV/AIDS recognizes KS as an AIDS-defining illness.HIV-associated KS is common among homosexual men; it is uncommon in countries where HIV is predominantly transmitted heterosexually. Because of this, despite high prevalence of HIV/AIDS in India, only 10 cases of KS exist in the published literature [Table 1]. This low prevalence of KS may be attributed to the low prevalence of HHV-8 in our country. [2] HIV-associated KS is usually asymptomatic, may be seen at any stage of HIV infection, even at normal CD4+ count [3] and CD4+ count is not a consistent prognostic indicator. In contrast to the other variants of KS, HIV associated KS can appear on any part of body with initial lesions frequently developing on the face, especially on the nose, eyelids, and ears-and on the trunk. Lesions of KS usually start as macule, progress to form papule, plaque and nodule. Sometimes pronounced lymphedema is observed in association with KS on the extremities, scrotum, penis, and face, especially when the eyelids are affected. Unusual cutaneous forms of KS include presentation like lichen planus, thrombophlebitic, telangiectatic, ecchymotic, pyogenicgranuloma, indurated plaque, keloidal, warty exophytic, and lymphangiomatous. [4] The lesions of AIDS-related KS, frequently involve the mucous membrane, lung, lymph node, and gastrointestinal tract. [5] The oral mucosa is the initial site of localization in 10-20% of all HIV-associated KS and is frequently located on the palate. Diagnosis of cutaneous KS is made on clinical ground and confirmed by histopathological examination. Prognosis of epidemic KS is related to the extent of KS, underlying immunosuppression, opportunistic infections, and treatment of HIV infection. [6] An excellent staging system has been developed by the National Institute of Allergy and Infectious Disease AIDS clinical trials group (ACTG). It distinguishes patients on the basis of tumor extent, immunological function and the presence or absence of systemic disease. [7] Good prognosis is expected when CD4 count is >200/mm 3 , only cutaneous involvement seen and no "B" symptoms (fever, weight loss, diarrhea). The fundamental basis for the treatment of AIDS-related KS is the suppression of HIV replication by starting antiretroviral treatment and treating the opportunistic infection. HAART can significantly decrease the incidence of KS, slow the rate of progression of KS and even result in regression of the preexistent disease. [8] Local treatment modalities include cryotherapy, intralesionalvinblastine or vincristine, laser and radiation therapy. It is useful when skin or mucosal lesions are few and there is no systemic involvement. Indications for systemic therapy include (1) visceral involvement, (2) extensive KS associated with lymphedema, (3) extensive and rapidly progressing KS and (4) failure to respond to local therapy.US FDA has approved liposomal anthracyclines (doxorubicin and daunorubicin) as the first line agent for KS. Paclitaxel appears to be more effective than liposomal anthracycline but because of the high toxicity paclitaxel is second line therapy. [9] Other systemic therapy includes interferon-alpha and a combination of chemotherapy. We report this case for its rarity in India and the occurrence of KS as the presenting manifestation of HIV disease
- Although HIV infection has been on a continuous and alarming rise in India, there are not many large clinical cohort studiesconducted on the neurologic manifestations of HIV infection. The insufficient number of cases and the inclusion in the analysisof outpatients limit the existing data from other studies. [7-11] In addition, because there are very few studies done on neurologicmanifestations in patients with subtype C HIV infection, which is more prevalent in India, this retrospective study wasundertaken to investigate the neurologic manifestations in HIV-infected inpatients admitted to our institute between 1993 and2003. Specifically, we evaluated the rising incidence of admissions due to HIV-related neurologic events and the impact of HIVinfection on neurologic abnormalities, characterized the diverse clinical neurologic presentations due to HIV infection, andcorrelated neurologic events with the CD4 count.A total of 7091 HIV-positive patients were identified at our institute during an 11-year period; of these, 5485 were eitheroutpatients or externally referred patients; the other 1606 were inpatients. Only the inpatients were included in the study.At our institute, many outpatients were later admitted as inpatients, and some inpatients who were discharged were asked toreturn as outpatients for further follow-up. Therefore, to avoid double counting of infected patients during the study period, HIVpositivepatients who were treated on an outpatient basis or who were referred to our institute were not included in the study. All1606 HIV-positive inpatients had been screened for various neurologic abnormalities, regardless of the unit or specialty throughwhich they were admitted. If an abnormality was found, the patient was included in the analysis.Patients' medical records were reviewed and evaluated for neurologic manifestations. Demographic information, including age,sex, and history of risk behavior, was collected for all patients. All presenting symptoms, neurologic signs, and diagnosticstudies (radiologic, pathologic, serologic, culture, biochemical, neuroimaging) were taken into consideration to stratify eachneurologic manifestation. Many of the patients had simultaneous occurrence of 2 or more syndromes; these were included asseparate cases.All serum samples were evaluated for HIV antibodies by enzyme-linked immunoassay. Each reactive specimen wasreevaluated by a second immunoassay. All of these were repeatedly HIV-reactive, and most were also confirmed by Westernblot analysis.CD4 testing in our institute was not started until 2000; therefore, data for only 123 (29.93%) of the HIV patients with neurologiccomplications were available. CD4 counts were determined using the FACS Count system (Becton & Dickinson), courtesy ofNACO, Government of India.Neurologic abnormalities are the first manifestations of HIV infection in 10% to 20% of symptomatic patients. [12] Neurologicproblems have been reported at all stages of HIV infection but are detected especially in advanced disease. [2,3,8] Many ofthese problems are secondary to the systemic effects of the disease or are caused by opportunistic infections or neoplasticdisease of the CNS. About one third of these patients show considerable morbidity with high mortality. [13]Neurologic complications of HIV infection often go unrecognized. Once diagnosed, neurologic symptoms and complicationsmay improve with treatment. Infections can be treated with antibiotics, but radiation therapy may be needed to treat AIDSrelatedcancers present in the brain or spinal cord.A fluctuating pattern in the prevalence of neurologic complications in HIV-infected patients was observed in our study: thenumber of cases increased for 5 consecutive years, then dropped off for 2 years, then rose again. The apparent increase from1993 to 1997 may have been related to more limited diagnostic techniques in the earlier era.HIV-associated dementia, also known as AIDS dementia complex (ADC), is one of the most frequent neurologic complicationsof HIV infection [14,15] and was seen in 8.03% of our cases. ADC in our patients had been uncovered by carefully obtaining apatient history and interview, which included questions about and assessments of short-term memory loss, concentrationdifficulties, attention and memory, and verbal fluency; digit symbol tests; and functional/physical function difficulty tests. CT orMRI scans of most of these patients showed diffuse cortical loss with prominent sulci. Because ADC has not previously beenreported in subtype C–infected persons, this finding should alert clinicians who treat subtype C–infected patients to monitor forthis complication.The ADC symptoms were di-vided into 3 main categories: cognitive, motor, and behavioral. Although the onset and progressionof HIV dementia varies, all the dementia cases were observed in the late stages of HIV infection, with affected patients having amean CD4 count of 92 cells/μL. The cognitive symptoms, primarily for getfulness associated with slowed mental abilities, wereseen in 7.05% of cases. Early motor signs, including loss of balance and leg weakness, were seen in 6.27%. The mostcommon be havioral symptoms—apathy and social withdrawal—were observed in 3.66% of cases.Among 1606 HIV-infected patients, 486 single or coexisting neurologic manifestations were found in 411 patients—an overallprevalence of 25.6%, which ranged from 15.8% in 1993 to 26.6% in 2003 (Figure 1).Meningitis as a neurologic manifestation constituted the majority of the complications in this study. Our analysis documentedthat tubercular meningitis was more common than cryptococcal meningitis (25.05% vs 10.95%), even though many studieshave shown the opposite. [8,11,16] In fact, tubercular meningitis was seen as the most prevalent type of meningitis.Of all the documented neurologic manifestations, meningitis and mass lesions related to TB were the most prevalent, becauseHIV infection predisposes to TB via immunosuppression. [17,18] Most of the TB diagnoses were based on the radiologic findings(CT or MRI scans or x-ray film results) and on the cerebrospinal fluid (CSF) analysis for adenosine deaminase activity (ADA),which suggested tubercular infections, even though many of these cases had negative acid-fast bacilli smear and Mantoux testresults. TB culture reports were not available, because TB culture testing takes a longer time. Almost all these patients hadshown a positive response to antituberculous treatment.Cryptococcal meningitis was the most common CNS fungal infection, a result similar to those of other studies. [8,16,19] Thisform of meningitis was associated with the most severe headaches and with fever, vomiting, altered mental status, and othermeningeal signs, but not with neck stiffness or photophobia. Cranial nerve deficits and seizures were seen in patients in thelate stages of infection and were seen as antemortem events. Diagnosis was based on detection of cryptococcal capsularantigen in the CSF (9.52%), positive India ink stain (40.48%), and cultures from blood and CSF (45.24%). CT and/or MRI scanssuggested cryptococcal meningitis, showing, for example, the presence of basilar inflammation, enlarged ventricles, andinfarcts in bilateral ganglia.Focal mass lesions of the CNS were present as a single lesion or multiple ring-enhancing lesions and were commonly seen inbasal ganglia. The mean CD4 count for patients with mass lesions was 61 cells/μL.The most common cause of focal brain lesions was toxoplasmosis (9.25%). Toxoplasma diffusely infects the whole CNS fromthe early stage of infection. [20] Because serologic testing may yield a false-negative result and because obtaining a brainbiopsy is difficult, diagnosis was based on the clinical and radiologic improvement in response to antitoxoplasmosis therapy, ashas been documented in other studies. [2,3,8,20,21] Testing for serum anti-ToxoplasmaIgG antibodies was positive in 31(81.58%) of the 38 patients in whom toxoplasmosis was diagnosed. (A low titer or an absence of antibody does not exclude thediagnosis.) The lack of IgM antibodies in most cases with the presence of IgG antibodies (in 31.43% of patients) shows thereactivation of a previously acquired endogenous infection.Tuberculoma was another common cause of lesions, although as mentioned earlier, diagnosis of this lesion is oftenpresumptive. ADA levels suggested TB in 83 (20.19%) of 411 cases. The Mantoux test was positive in 2.09%, and culture forMycobacterium tuberculosis was positive in 1.83%.The incidence of primary CNS lymphoma was 6.08% (25 of 411 cases). Although previously considered a rare disorder, thislymphoma is observed to have increased in association with HIV infection, as seen in other studies. [21,22] It occurs in up to2.4% of all HIV-infected persons and is strongly associated with the Epstein-Barr virus.HIV-associated sensory neuropathy was seen as a prevalent neuropathy (1.57%). Of all toxic neuropathy cases, 0.52% couldhave been caused by the increased use of potentially neurotoxic antiretroviral agents.Myelopathy is a spinal cord dysfunction that is mostly caused by spinal stenosis; a slowly progressive, yet painless, gaitdisturbance and lower extremity sensory complaints develop. Although myelopathy is not directly caused by HIV infection, itmay be indirectly due to the infiltration of HIV-infected cells releasing cytotoxic cytokines or to an impaired ability to usevitamin B 12 as a source of methionine for myelin maintenance. The most common spinal cord involvement in this study wascompressive myelopathy secondary to Pott disease (tuberculous spon dylitis), although acute myelopathy was also frequentlyobserved, as documented in other research. [5] Vitamin B 12 deficiency was observed in 4 of these cases.Data from this study conducted in southern India show that there has been a fluctuating pattern in the prevalence of HIVpositivepersons with neurologic manifestations. Neurologic manifestations are common in HIV-infected persons: there hasbeen considerable morbidity and increased mortality due to neurologic complications, which can affect the nervous system atall levels and at all stages of HIV disease. In addition, the nervous system can be affected by multiple complications at thesame time, which adds to the difficulty of diagnosis. Because of the varying clinical presentations of neurologic complicationsin HIV disease, obtaining a precise diagnosis is critical for determining the specific therapeutic approach and proper diseasemanagement. This study also revealed that the risk of various neurologic abnormalities can usually be de fined by the CD4count, thus stressing the need for routine assessment of CD4 counts in HIV-positive patients with neurologic manifestations.
- Neurologic Manifestations of the hiv: An Indian StudyAbstractContextHIV-1 is a neurotropic virus. In a resource-limited country such as India, large populations of affected patients now have access to adequate chemoprophylaxis for opportunistic infections (OIs), allowing them to live longer. Unfortunately the poor availability of highly active antiretroviral therapy (HAART) has allowed viral replication to proceed unchecked. This has resulted in an increase in the debilitating neurologic manifestations directly mediated by the virus.ObjectiveThe main objective of this study was to identify and describe in detail the direct neurologic manifestations of HIV-1 in antiretroviral treatment (ART)-naive, HIV-infected patients (excluding the neurologic manifestations produced by opportunistic pathogens).DesignThree hundred successive cases of HIV-1 infected, ART-naive patients with neurologic manifestations were studied over a 3-year period. Each case was studied in detail to identify and then exclude manifestations due to opportunistic pathogens. The remaining cases were then analyzed specially in regard to their occurrence and the degree of immune suppression (CD4+ cell counts).Setting and PatientsThe study was carried out in an apex, tertiary, referral care center for HIV/AIDS in India. All patients were admitted for a detailed analysis.No interventions were carried out, as this was an observational study.ResultsOf the 300 cases, 67 (22.3%) had neurologic manifestations due to the direct effects of HIV-1. The HIV infection involved the neuroaxis at all levels. The distribution of cases showed that the region most commonly involved was the brain (50.7%). The manifestations included stroke syndromes (29.8%), demyelinating illnesses (5.9%), AIDS dementia complex (5.9%), and venous sinus thrombosis (4.4%). The other manifestations seen were peripheral neuropathies (35.8% of cases), spinal cord pathologies (5.9% of cases), radiculopathies (4.4% of cases), and a single case of myopathy. The onset of occurrence of these diseases and their progression were then correlated with the CD4+ cell counts.ConclusionsHIV infection is responsible for a large number of nonopportunistic neurologic manifestations that occur across a large immune spectrum. During the early course of the disease, the polyclonal hypergammaglobulinemia induced by the virus results in demyelinating diseases of the central- and peripheral nervous systems (CNS and PNS). As the HIV infection progresses, the direct toxic effects of the virus unfold, directly damaging the CNS and PNS, resulting in protean clinical manifestations.DiscussionThis study has shown that HIV-1 is indeed a neurotropic virus, which can produce a large variety of neurologic manifestations affecting all levels of the neuroaxis. Although OIs of the nervous system are still by far the most commonly seen manifestations,[1] the availability of good prophylactic and therapeutic medications for these OIs has meant that more features of direct HIV neuroinvolvement are being seen.The HIV viral RNA and other component proteins have been demonstrated in neural tissues by processes such as in situ hybridization and immunocytochemistry.[1,2] They have predominantly been isolated from the microglial cells, giant cells, and capillary endothelial cells.[3] The virus appears to enter the brain via the infected macrophages, a pathogenic mechanism described by the “Trojan horse hypothesis.”[4] The total number of infected cells is low, but the virus appears to induce widespread neuronal dysfunction predominantly through cytokine release.Strokes and TIAs are commonly seen in patients with HIV disease. Although a large number of them are due to OIs, in approximately half there is no cause discernable apart from the HIV disease itself. Clinical evidence of strokes and TIAs has been found in approximately 1.5% of patients with advanced HIV disease.[5,6] Brew and colleagues[5] found the mean CD4+ cell count to be 130 80 cells/mcL, with most having CD4+ cell counts < 50 cells/mcL. These figures were derived from studies carried out in ART-naive patients, very similar to the population base in our country. In our study, 29.8% of non-OI manifestations were stroke syndromes. Ten patients (50%) had a CD4+ cell count between 200 and 500 cells/mcL, whereas 8 cases had a CD4+ cell count between 100 and 200 cells/mcL. The mean CD4+ cell count in the study was found to be 212 cells/mcL. Seventy-five percent of the patients had a young stroke, aptly proving the fact that HIV disease results in a prothrombotic state. This prothrombotic state is due to a complex mix of effects of anticardiolipin antibodies, low protein S levels, and altered heparin cofactor II levels.[5–9] In addition to this, vasculitis produced by the virus itself tends to be prothrombotic due to the endothelial dysfunction, resulting in a significant overlap between the two. In advanced HIV infection, excessive inappropriate elaboration of TNF alpha and interleukin-1 add to the thrombophilia.[10]In HIV disease most cases of demyelination involve the spinal cord. In this study we found 4 cases (5%) that presented with CNS demyelination. Berger and coworkers[11] reported 7 cases of multiple sclerosis-like illness in HIV-positive patients. It is found to occur in the early phases of the retroviral infection, where there is a polyclonal hypergammaglobulinemia. The antibodies cross-react with myelin in the CNS, resulting in the demyelination. As the HIV disease progresses, with advancing immune deficiency, it is observed that there is an improvement in the clinical status of these patients.[12] This probably arises from the fact that, with advancing immune deficiency, the ability of the body to mount an immune-mediated response progressively gets disabled. In this study, 2 out of 4 patients had marked improvement. One of them had a relapsing-remitting form of disease, and 1 died due to Pneumocystiscarinii pneumonia.In this study we found that 5% of the non-OI cases were due to the AIDS dementia complex (ADC). Increasingly more number of cases of ADC are going to be seen, due to the availability of good prophylaxis and therapy for OIs. Studies from western countries have shown that 20% to 30% of patients with advanced HIV infection go on to develop ADC.[13]Wadia and associates[14] reported 21 cases of ADC of a total of 481 cases (4.3%) of HIV patients with neurologic manifestations. The advent of antiretroviral therapy (HAART) in western countries has led to significant reduction in the incidence of ADC.[15]The bulk of the cases in this study, 38%, were constituted by HIV neuropathy. It has been shown in different studies that between 10% and 35% of HIV-infected individuals develop a neuropathy that can be ascribed to the HIV infection itself.[16,17]Histopathologic abnormalities in peripheral nerves have been found in over 95% of patients dying with AIDS.[18] Neuropathy is found to occur at all stages of HIV infection. During seroconversion we saw cases of facial neuropathy and acute inflammatory demyelinating neuropathies. As the disease advanced, mononeuritis multiplex, secondary to vasculitis and polyneuritis cranialis, were evident. Finally, with advanced HIV disease, there were cases of distal painful predominantly sensory neuropathy. There were 8 cases of the Guillain-Barr syndrome. One important feature seen in all of these patients with Guillain-Barr syndrome was that their CSF analysis at the end of the first week did not show the classic albuminocytologic dissociation. All studies have shown either a raised protein level and or a mononuclear pleocytosis.[19,20]Cornblath and colleagues[19] found the mean CSF cell count to be 23 cells/mcL, with a maximum of 43 cells/mcL. In our study, all of the patients had a slightly elevated CSF protein content, and the CD4+ cell count ranged from 0 to 30 cells/mcL, with a mean of 15 cells/mcL.By performing this study, we have realized that this disease is constantly evolving and placing new challenges in front of the physician and the society. Right from its detection in 1981 up to the new millennium, every single year that has passed by has seen this virus evolve and remain elusive to medical therapy. Billions of dollars have been spent in both prevention and cure, but still the epidemic continues to grow, especially in third-world countries. In the year 2000, the United Nations Security Council discussed this disease as an issue of global security in their annual meeting. The youth of countries across the globe were succumbing to the effects of the HIV infection destroying the economic and social fabric of these countries.In the current world scenario, with the advent of HAART and effective chemoprophylaxis for OIs, there has been a significant impact on the disease. However, as the incidence of the opportunistic manifestations was reduced by chemoprophylaxis, an entire spectrum of non-OI manifestations and drug related toxicities evolved.From a physician's perspective, these non-OI manifestations are difficult to diagnose because of the lack of specific tests and limited knowledge that is available. Most of these non-OI neurologic manifestations are crippling, imposing huge burdens on the patient's family and the healthcare system.The treatments of most of these manifestations are limited. The initial immune-related phenomenon, such as the Guillain-Barr syndrome, could be dealt with by using immune globulins, plasmapheresis, and steroids. The costs of immune globulins are exorbitant, and most institutions do not offer plasmapheresis facilities to HIV-positive patients. Use of corticosteroids in HIV patients for both Guillain-Barr syndrome and demyelinating illnesses always has to be done with apprehension, as these patients are already immune-compromised and prone to infection.The ideal way to assess the relationship between the non-OI neurologic manifestations and HIV disease progression is by estimating the plasma and CSF viral loads. In India today the only affordable methodology is the estimation of the CD4+ cell counts. There are laboratories that estimate the plasma viral load, but the costs have proven to be prohibitive. As far as the CSF viral load is concerned, this technology remains to be introduced in the country.
- Common: CMV encephalitis is a reactivation of latent CMV infection - features cell death in meninges and peri-ventricular areaOften associated with a CMV retinitisRapidly progressing; responds well to treatment if caught in time otherwise responds poorlyTreatment is usually IV ganciclovir, valganciclovir, foscarnet, cidofovir – these drugs can be quite toxicPresentations vary, however usually involve confusion, headache, deliriumCan have focal neurology, cranial nerve deficitsOther encephalitis presentations include HSV (Herpes Simplex Virus) and VZV (Varicellar Zoster Virus)
- Caused by Cryptococcus neoformans (rarely, Cryptococcus neoformans var. gattii)Most cases seen in patients with CD4 count <50 cells/µL5-8% prevalence among HIV-infected patients in developed countries before widespread use of effective ARTIncidence much lower with use of ART
- Profile of tuberculous meningitis with or without HIV infection and the predicators of adverse outcome SK BandyopadhyayI; R BandyopadhyayII; A DuttaIIIIDepartment of Medicine Nil RatanSircar Medical College and Hospital, 138, A JC Bose Road Kolkata 700014 India IIDepartment of Pathology, Medical College, 88 College Street Kolkata 700073, India IIIDepartment of Medicine Nil RatanSircar Medical College and Hospital, 138 AJC Bose Road Kolkata 700014 IndiaDISCUSSIONIn this study, the aim was to analyse clinical, radiological and CSF findings in TBM cases to evaluate whether HIV infection significantly influences the characteristic findings. The results showed that most of the patients who were HIV-positive were in the AIDS portion of the HIV spectrum (CD4 count < 200/cmm). They failed to mount a significant rise in temperature owing to underlying immunosuppression and had seizures more commonly due to the presence of a gamut of subclinical CNS diseases (opportunistic infections as well as noninfectious causes). Also hydrocephalus and cerebral infarction were more common in them. Co-existing HIV encephalopathy, opportunistic infections and extensive vasculopathy probably contributed to such radiological findings. Notwithstanding previous reports (2), HIV infection in the patients did not significantly altered the presenting CSF findings.Berenguer et al reported that infection with HIV does not appear to change the clinical manifestations or the outcome of TBM (3). Similar results were found by Bossi et al (4). In a study from South Africa, clinical and CSF findings were similar in HIV-infected and uninfected TBM patients but ventricular dilatation and infarcts were more frequent in patients who were HIV-positive and GCS was a better indicator of prognosis than CD4 count (5). Similar to our observations, one group from India found that many of the clinical, radiological and pathological features of TBM in patients who were HIV-positive was distinctly different from those without HIV infection (6).The presence of hydrocephalus is an established risk factor for poor outcome in TBM patients while a low GCS score is a predictor of 6-month outcome in HIV-infected patients (2, 7). In addition, disease severity at admission, presence of focal weakness, prolonged somatosensory evoked potential and absence of corticosteroid use had been shown to be associated with death or poor prognosis in various analyses (7, 8).
- A demyelinating lesion of sub cortical white matter (Cerebral hemisphere predilection to parieto-occipital area) , cerebellum , BG, thalamus, brainstem & spinal cordHAART –Regression of lesion and -Prolonged survival > 2.5 yearsTrial: no clear benefit by cidofovir, IFN , topoisomerase inhibitor, cytosine arabinosideMedian survival in HIV pts + PML ~2.6 monthsIn patients on HAART 1year survival has increased from10%50%Paradoxical worsening has been seen with initiation of HAART as IRISSpontaneous remission -8%Favorable out come Baseline CD4 > 100/ul, Maintenance of Viral load < 500 copies/ml ( baseline doesn’t have independent predictive value of survival)PrognosisMedian survival in HIV pts + PML ~2.6 monthsIn patients on HAART 1year survival has increased from10%50%Paradoxical worsening has been seen with initiation of HAART as IRISSpontaneous remission -8%Favorable out come Baseline CD4 > 100/ulMaintenance of Viral load < 500 copies/ml ( baseline doesn’t have independent predictive value of survival)**One of the few OIs that continues to occur with some frequency despite widespread use of HAART:
- 1000-4000 times more common in HIV+ population than in immunocompetent populationDoesn’t correlate with low CD4 countsPathogenesis not fully understood but known to be linked to the Epstein-Barr VirusThought that long term low level immune system damage may be contributing factorIs generally non-Hodgkin’s B-cell type with high mitotic rate; tumours usually double in size in 14 days. (can also be a Burkitt or more rarely a Primary Effusion Lymphoma)Can be multifocal (50%) and appear in uncommon locations with greater frequency than in non-HIV populationStudies have average survival rates from diagnosis between 3 and 24 monthsMay be treated actively or palliatively with radiotherapy (usually palliative) or high dose methotrexate (chemo)Disagreement between researchers whether discontinuing or continuing ARVs throughout treatment is most beneficialTherapy input is usually initially around advice / treatment to help maintain function / independence and planning for deterioration / palliative approach
- Thought that HIV cells can lead to axonal degeneration (resulting in DSPNs)Thought that HIV can lead to inflammation / demylination (resulting in inflammatory demyelinating neuropathies)DISTAL SYMMETRICAL POLYNEUROPATHY ( DSPN )Clinical: Painful paresthesias of feet and soles, shooting leg pains, numbness; weakness, subjective or mildStocking-glove sensory loss, decreased vibratory sense in ankles, normal position sense, absent or reduced ankle jerksDISTAL SYMMETRICAL POLYNEUROPATHY (DSPN)Most common neuropathy of HIV infection and may be disablingPrevalence increases with disease stage, most prevalent in chronic HIV infection or advanced diseaseConcurrent conditions may include myelopathy, dementia, constitutional symptoms and weight lossDSPN: DIFFERENTIAL DIAGNOSISHIV- relatedDrug or treatment relatedMetabolic or Nutritional disorderSecondary infection Unrelated to HIV illnessDSPN: ETIOLOGYInfectious: HIV, CMV, Hepatitis virus, MAI, other infectionsNutritional: B12 deficiency, Acetyl carnitine deficiencyAuto-immune: Anti-sulfatide, anti-Mag and other auto-antibodiesNeurotoxic drugs: Antiretrovirals, INH, chemotherapy, othersAUTONOMIC NEUROPATHYClinical : Orthostatic hypotension; impotence, diarrheaEtiology: Presumed HIV-related sympathetic ganglioneuropathyImportant as potential cause of sudden cardiac arrest during proceduresPROGRESSIVE POLYRADICULOPATHYClinical: Progressive paraparesis, areflexia, urinary retention, ascending sensory lossEtiology: CytomegalovirusDiagnosis: Polymorphonuclear pleocytosis may be present in cerebrospinal fluid; EMG/NCV, acute denervation; CSF PCR and positive blood cultures help in diagnosis.NEUROPATHY IN HIV INFECTION: EVALUATIONStage disease: CD4 count; viral loadFamily HistoryEnvironmental or toxic exposureOther: Tick bite or exposure risk; malnutrition and weight lossMedication historyNEUROPATHY IN HIV INFECTION: EVALUATIONSerology: Cytomegalovirus (CMV), Lyme, Hepatitis, MAG, sulfatide, GM1 gangliosideCultures: Blood for CMV, MAI; rectal and throat swab for CMVOther: B12, thyroid function, heavy metalsNEUROPATHY IN HIV INFECTION: EVALUATIONCerebrospinal fluid: cell count; glucose; protein; VDRL; cultures for bacteria, fungus, viruses and acid fast bacilli (AFB, includes MAI); Lyme serology. Polymerase chain reaction (PCR) for CMV, Lyme or AFB as indicated.Electromyography, nerve conduction; nerve biopsy in select cases NEUROPATHY IN HIV INFECTION: TREATMENTImmune therapy: useful for AIDP/CIDP and may control disabling pain of DSPNPlasmapharesisImmune globulin*SteroidsNEUROPATHY AND HIV INFECTION: TREATMENTPain treatmentAnticonvulsants: Carbamazepine, phenytoin, gabapentin, lamotrigineTricyclic antidepressants: amitriptyline, nortriptylineMexilitineOpioidsNEUROPATHY IN HIV INFECTION: TREATMENTNeuropathy due to secondary infection (CMV, MAI or Lyme) responds to specific anti-viral or antibiotic therapy Failed therapies: Peptide T; nerve growth factor Lumbosacral Polyradiculopathy