Intimal hyperplasia
- 1. F2 Parach Sirisriro 1st April 2019 INTIMAL HYPERPLASIA
- 2. REFERENCE Textbook 1. Rutherford's Vascular Surgery and Endovascular Therapy 9th edition , Chapter 47, 589-601.e 2. Vascular and Endovascular Surgery: A Companion to Specialist Surgical Practice, 6th Edition , Chapter 7, 83-101.e
- 3. OUTLINE • RESPONSE OF THE ARTERY TO INJURY - Hemodynamics - Systemic Vascular Diseases - Intravascular Bare Metal Stents - Intravascular Drug-Eluting Stents - Intravascular Drug-Eluting Balloons • RESPONSE OF VEIN TO INJURY • HEALING RESPONSE OF THE PROSTHETIC GRAFT • INTIMAL HYPERPLASIA AND DIALYSIS ACCESS • CONCLUSION
- 4. DEFINITION • The abnormal migration and proliferation of vascular smooth muscle cells with the associated deposition of extracellular connective tissue matrix • followed by remodeling of this new tissue 1. Rutherford's Vascular Surgery and Endovascular Therapy 9th edition , Chapter 47, 589-601.e
- 5. STAGE OF INTIMAL HYPERPLASIA 1. Rutherford's Vascular Surgery and Endovascular Therapy 9th edition , Chapter 47, 589-601.e
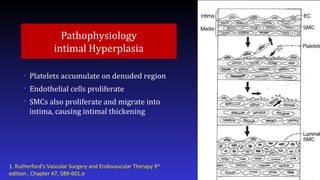
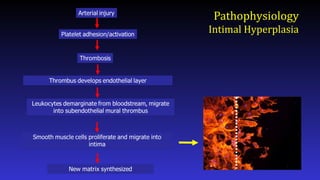
- 6. Pathophysiology intimal Hyperplasia • Platelets accumulate on denuded region • Endothelial cells proliferate • SMCs also proliferate and migrate into intima, causing intimal thickening 1. Rutherford's Vascular Surgery and Endovascular Therapy 9th edition , Chapter 47, 589-601.e
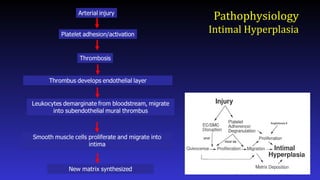
- 8. Pathophysiology Intimal Hyperplasia Conde et.al. Cath & Cardiovasc Int. 2003;60:236
- 9. Pathophysiology Intimal Hyperplasia Conde et.al. Cath & Cardiovasc Int. 2003;60:236
- 10. Pathophysiology Intimal Hyperplasia Conde et.al. Cath & Cardiovasc Int. 2003;60:236
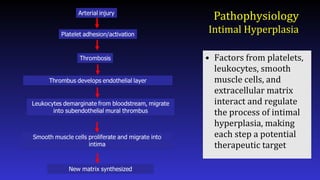
- 12. Pathophysiology Intimal Hyperplasia • Factors from platelets, leukocytes, smooth muscle cells, and extracellular matrix interact and regulate the process of intimal hyperplasia, making each step a potential therapeutic target
- 14. RESPONSE OF THE ARTERY TO INJURY ANGIOPLASTY
- 15. RESPONSE OF THE ARTERY TO INJURY REMODELING
- 16. Hemodynamics - Changes in hemodynamic parameters affect the arterial structure of both normal and diseased vessels. - Blood flow (closely associated with shear stress) is associated with the formation of intimal hyperplasia In an experimental study, Hehrlein confirmed that reduced vascular runoff after angioplasty results in the increased development of intimal hyperplasia
- 17. Systemic Vascular Diseases - Exposure to cigarette smoke increases the development of experimental intimal hyperplasia by two fold. - Cholesterol reduction therapies with statins have been shown in some studies to reduce the development of restenosis - Diabetes is a predictor for restenosis. - Restenotic plaques in diabetic patients is composed of atherosclerotic plaque . - This might suggest that recoil/remodeling may be predominant.
- 18. CONSEQUENCE AND CURE OF ANGIOPLASTY
- 19. - After balloon angioplasty, there is thrombus formation, intimal hyperplasia development, elastic recoil, and negative remodeling. - In contrast, after stent placement, elastic recoil and negative remodeling are eliminated and thrombus formation followed by intimal hyperplasia development are the main contributors to “Instent restenosis” CONSEQUENCE AND CURE OF ANGIOPLASTY
- 20. Mechanism of Instent restenosis • A stent is generally used if – the result of balloon angioplasty is technically unsatisfactory – if there is arterial occlusion, immediate elastic recoil, dissection, or restenosis • Four categories of in-stent restenosis have been defined: (1) focal (≤10-mm length) (2) diffuse (>10-mm length) (3) proliferative (>10-mm length and extending outside the stent) (4) occlusion
- 26. The chief benefit cited for DEBs is -Avoidance of additional metal and polymer barriers, which may disrupt or hinder vascular healing -DEB-treated vessels show - delayed vascular healing characterized by dose-dependent increases in fibrin deposition, - delayed re-endothelialization, - lower number of neointimal cells, - increased medial VSMC loss Intravascular Drug-Eluting Balloons
- 27. RESPONSE OF THE VEIN TO INJURY
- 28. Healing response of prosthesis graft
- 29. Intimal Hyperplasia and Dialysis Access - AVBG : The anastomoses appear to be the areas of maximal intimal hyperplasia as a result of surgical trauma and the presence of flow disturbance. - The majority of stenoses occur at the venous anastomoses and within 1 cm of the anastomosis AVF : Five anatomic stenotic lesions in arteriovenous fistula grafts have been categorized: stenosis in the draining vein proximal to the venous anastomosis (36%), stenosis in the central vein (24%), stenosis at the venous anastomosis (25%), stenosis at the arterial anastomosis (11%), and intragraft hyperplasia (4%).
- 30. Treatment Vascular reconstruction • Principal way to salvage failing grafts • Based on assumption that renewed or continued intimal thickening is unlikely • With regular follow-up, stenoses in vein grafts may be discovered prior to graft thrombosis • If these lesions are reconstructed in time, long-term outcome is generally good
- 31. Conclusion Intimal hyperplasia Intimal hyperplasia is a complex response to injury. Vascular reconstruction is effective in salvage of vein grafts if performed in time Antiplatelet agents are effective if given at or within a short time of surgery Development of effective therapy requires an understanding of the underlying pathophysiology.
Editor's Notes
- Stage if intimal hyperplasia Hyperacute minutes to hours Acute Hours to week Chronic Wks to months
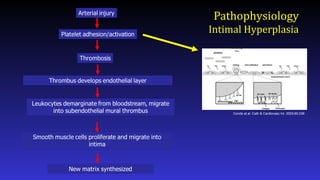
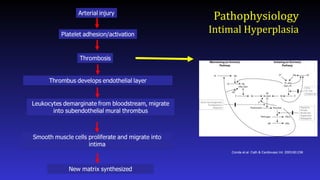
- Here is a simplified schematic showing some of the steps in the complex process of neointimal formation. Important players are platelets, thrombosis, inflammation, smooth muscle proliferation, and matrix synthesis.
- After arterial injury, platelets roll onto damaged areas, adhere, and are activated. They elaborate factors that contribute to thrombosis, inflammation, and smooth muscle migration and proliferation.
- Targeting of thrombosis has also been attempted, to somewhat disappointing results
- What about inflammation? Leukocytes including monocytes and neutrophils play a role in neointimal hyperplasia by elaborating factors that stimulate smooth muscle cell proliferation and migration. Leukocytes roll onto the damages vessel wall, adhere, and extravasate. Adhesion molecules and selectins play important roles in this process.
- Finally, smooth muscle cell migration and proliferation provides an attractive target for drug therapy as much of the neointimal is derived from smooth muscle.
- Finally, smooth muscle cell migration and proliferation provides an attractive target for drug therapy as much of the neointimal is derived from smooth muscle.
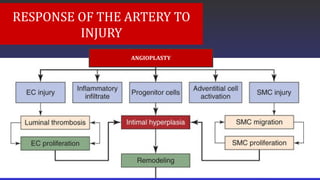
- Pathobiology of the Injury Response After Angioplasty. Flow diagram demonstrating the key elements in the vessel's responseto arterial injury. Endothelial cell (EC) injury leads to luminal thrombosis, inflammatory cell infiltration, cellular proliferation, andclearance of the thrombotic material on the surface with development of a neoendothelium. Injury to smooth muscle cells (SMCs) andadventitial cells leads to cell proliferation and migration. Progenitor cells are recruited to the vessel wall. With the migration of proliferationof SMC and adventitial cells, the appearance of progenitor cells and the deposition of extracellular matrix,
- intimal hyperplasia develops.Over time this lesion remodels and may either remain stable or demonstrate positive remodeling with an in luminal diameter ornegative remodeling with a decrease in luminal diameter. These chronic changes in the intimal lesion can lead to continued patency,restenosis, or occlusion.
- Consequences and Cures of Angioplasty. Flow diagram demonstrating the outcomes of a vessel's response to balloonangioplasty and the therapeutic maneuvers to correct the adverse outcomes. If a technical failure occurs after angioplasty due to elasticrecoil or dissection, a stent is placed. If there is concern for the development of intimal hyperplasia, brachytherapy or cryotherapy maybe applied. Sudden occlusion is corrected with thrombolysis or primary stenting. Remodeling will be influenced by placement ofstents, drug-eluting stents, brachytherapy, or cryotherapy.
- 4 month f/u
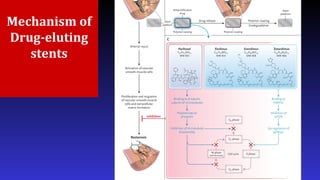
- Sirolimus-, paclitaxel-, and more recently, ABT-578–eluting stents are commercially available
- Sirolimus : antiproliferative drugs. (B) The mode of action of sirolimus: sirolimus binds to the FK-bindingprotein 12 (FKBP12), which in turn inhibits the mammalian target of rapamycin (mTOR) pathway. This subsequently prevents the downregulation of the cell division kinase inhibitor p27kip1, thereby inhibiting cell division between phases G1 and S1 of the cell cycle. Sirolimus has a wide dose range, is cytostatic as opposed to cytotoxic, and has additional anti-inflammatory properties through its inhibition of interleukin-2, which reduces T- and B-cell activation. DNA deoxyribonucleic acid mode of action of paclitaxel, an extract derived from the bark of the Taxus brevifolia (Pacific Yew) tree. Paclitaxel inhibits smooth muscle cell proliferation through the stabilization of microtubules, and thereby inhibits cell division.
- . • Conventional semi-compliant angioplasty balloons • Covered with an anti-proliferative drug which is released into the vessel wall during inflation of the balloon • Inflation usually at nominal pressures with a specific minimal inflation time • Active substance on the DEB is lipophilic with high absorption rate through vessel wall (to compensate for the short period of contact between the inflated balloon and the vessel wall)
- .
- Pathobiology of the Vein Graft Response to Implantation. Flow diagram demonstrating the key elements in the vein's response to insertion into the arterial circulation. Denudation of the endothelium is dependent on the degree of implantation injury. Endothelial cell (EC) injury leads to luminal thrombosis, inflammatory cell infiltration, cellular proliferation, and clearance of the thrombotic material on thesurface with restoration of the endothelium. If this fails to progress adequately, graft thrombosis may occur. Injury to smooth muscle cells(SMCs) leads to cell proliferation and migration. Progenitor cells are recruited to the vessel wall. With the migration of proliferation of SMC,the appearance of progenitor cells and the deposition of extracellular matrix (ECM), intimal hyperplasia develops to reestablish thetangential stress across the wall. Over time this lesion remodels and may produce a stenotic lesion due to the bulk of neointima or due tonegative remodeling restenosis. This may result in graft occlusion
- . Pathobiology of the Prosthetic Graft Response After Implantation. Flow diagram demonstrating the key elements of thebody's response to implantation of a prosthetic graft into the arterial or venous circulation. Luminal thrombosis and adventitial infiltration areassociated with an inflammatory response and recruitment of progenitor cells. Injury to the endothelial cells (ECs) and smoothmuscle cells (SMCs) at either the venous or arterial anastomosis leads to the development of intimal hyperplasia within the native vessel andingrowth into the prosthetic conduit as a result of EC and SMC migration and proliferation occurs with the laying down of anextracellular matrix (ECM). Over time these lesions remodel and may produce a multifocal stenotic lesion at the anastomoses and in thefloor of the recipient vessel.
- .
- More generally in conclusion, I will say that: