cls 10 sci chp 2 ppt.pdf
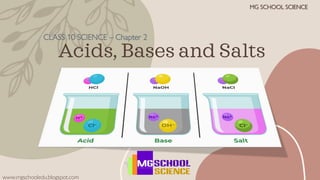
- 1. Acids, Bases and Salts CLASS 10 SCIENCE – Chapter 2 www.mgschooledu.blogspot.com MG SCHOOL SCIENCE
- 2. Tastes of some common edible substances :- Edible substances have different tastes. Some have sour taste, some have bitter taste, some have sweet taste and some have salty taste. www.mgschooledu.blogspot.com MG SCHOOL SCIENCE 2 SI No Substance Taste 1 Lemon juice sour 2 Orange juice Sour/sweet 3 Vinegar Sour 4 Curd Sour 5 Tamarind Sour 6 Sugar Sweet 7 Common salt Salty 8 Amla Sour 9 Baking soda Bitter 10 Grapes Sour/sweet 11 Unripe mango Sour 12 glucose Sweet Chapter 2 Acids, Bases and Salts
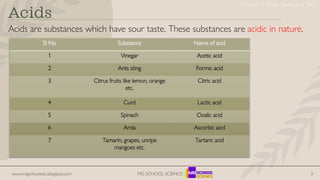
- 3. www.mgschooledu.blogspot.com MG SCHOOL SCIENCE 3 Acids Acids are substances which have sour taste. These substances are acidic in nature. SI No Substance Name of acid 1 Vinegar Acetic acid 2 Ants sting Formic acid 3 Citrus fruits like lemon, orange etc. Citric acid 4 Curd Lactic acid 5 Spinach Oxalic acid 6 Amla Ascorbic aicd 7 Tamarin, grapes, unripe mangoes etc. Tartaric acid Chapter 2 Acids, Bases and Salts
- 4. Bases are substances which have bitter taste and have a soapy touch. Theses substances are basic nature. 4 Bases SI No substance Name of base 1 Lime water Calcium hydroxide 2 Window cleaner Ammonium hydroxide 3 Soap Sodium hydroxide potassium hydroxide 4 Milk of magnesia Magnesium hydroxide www.mgschooledu.blogspot.com MG SCHOOL SCIENCE Chapter 2 Acids, Bases and Salts
- 5. Substances which are neither acidic nor basic are called neutral substances. Ex: sugar solution, salt solution, distilled water etc. 5 Neutral substances Substances which change their color in acidic and basic solutions are called indicators. Ex: litmus, turmeric, China rose petals are some naturally occurring indicators. Indicators www.mgschooledu.blogspot.com MG SCHOOL SCIENCE litmus paper turmeric China rose Chapter 2 Acids, Bases and Salts
- 6. 1. Natural indicators 2. Synthetic indicators 3. Olfactory indicators Natural indicators: Found in nature in plants. Examples: Litmus, red cabbage leaves extract, flowers of hydrangea plant, turmeric. 6 Types of Indicators and its properties red cabbage leaves turmeric flowers of hydrangea www.mgschooledu.blogspot.com MG SCHOOL SCIENCE Chapter 2 Acids, Bases and Salts
- 7. Litmus as indicator • Litmus is a natural indicator obtained from lichens. It is available in the form of solution as blue litmus solution and red litmus solution or as strips of paper as blue litmus paper and red litmus solution. • In distilled water its color is purple. In acidic solution it turns red and in basic solution it turns blue. 7 Natural indicators www.mgschooledu.blogspot.com MG SCHOOL SCIENCE lichens litmus paper Chapter 2 Acids, Bases and Salts
- 8. 8 Synthetic indicators: These are chemical substances. Examples: Methyl orange, phenolphthalein. Types of Indicators and its properties www.mgschooledu.blogspot.com MG SCHOOL SCIENCE Chapter 2 Acids, Bases and Salts
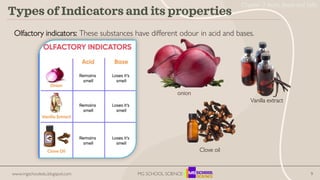
- 9. 9 Types of Indicators and its properties Olfactory indicators: These substances have different odour in acid and bases. Vanilla extract onion Clove oil www.mgschooledu.blogspot.com MG SCHOOL SCIENCE Chapter 2 Acids, Bases and Salts
- 10. • The term 'acid' has been derived from the Latin word, 'acidus' which means sour. • Acids have sour taste. • They turn blue litmus solution red. • They give H+ ions in aqueous solution. Strong Acids: HCI, H2S04 , HNO3 Weak Acids: CH3COOH, Oxalic acid, Lactic acid Concentrated Acids: More amount of acid + Less amount of water Dilute Acids: More amount of water + Less amount of acid 10 Properties of Acids www.mgschooledu.blogspot.com MG SCHOOL SCIENCE Chapter 2 Acids, Bases and Salts
- 11. 11 • These are the substances which are bitter in taste and soapy in touch. • They turn red litmus solution blue. • They give OH- ions in aqueous solution. Strong Bases: NaOH, KOH, Ca(OH)2 Weak Bases: NH4OH Alkalis: These are bases which are soluble in water. Examples: NaOH, KOH, Ca(OH)2. Properties of Acids www.mgschooledu.blogspot.com MG SCHOOL SCIENCE Chapter 2 Acids, Bases and Salts
- 12. 12 Reaction of Acids with Metals: Acids react with metal to form metal salt and releases Hydrogen Gas. Acid + Metal → Salt + Hydrogen Gas Example: Zinc granules react with dilute Hydrochloric acid in a test tube. 2HCl + Zn → ZnCl2 + H2 Hydrogen gas released can be tested by bringing burning candle near gas bubbles, it burst with pop sound. www.mgschooledu.blogspot.com MG SCHOOL SCIENCE Chapter 2 Acids, Bases and Salts
- 13. 13 Bases react with metal to evolve hydrogen Gas. Also, note that all metals do not react with bases. The metal must be more reactive than the metals present in the base for the reaction to take place. Base + Metal → Salt + Hydrogen gas Example: Zinc granules react with NaOH solution to form sodium zincate and evolve hydrogen gas. 2NaOH + Zn → Na2ZnO2 + H2 Reaction of Base with Metals: www.mgschooledu.blogspot.com MG SCHOOL SCIENCE Chapter 2 Acids, Bases and Salts
- 14. Acids reacts with Metal Carbonates and Metal Hydrogen carbonates to form Salt, Carbon dioxide and water. Metal carbonate/Metal hydrogen carbonate + Acid → Salt + Carbon dioxide + Water Examples: (i) 2HCl + Na2CO3 → 2NaCl + CO2 + H2O (ii) HCl + NaHCO3 → NaCl + CO2 + H2O CO2 can be tested by passing it through lime water. It turns lime water milky. Ca(OH)2 + CO2 → CaCO3 + H2O When excess CO2 is passed, milkiness disappears. CaCO3 + CO2 + H2O → Ca(HCO)3 ➢Bases do not react with Metal Carbonates and Metal Hydrogen carbonates. Base + Metal Carbonate/Metal Hydrogen Carbonate → No Reaction 14 Reaction of Acids with Metal Carbonates and Metal Hydrogen carbonates www.mgschooledu.blogspot.com MG SCHOOL SCIENCE Chapter 2 Acids, Bases and Salts
- 15. Acids and Bases react to form salt and water. Acid + Base → Salt + Water Neutralization Reaction: Reaction of acid with a base is called as neutralization reaction. Example: HCl + NaOH → NaCl + H2O Strong Acid + Weak Base → Acidic salt + H2O Weak Acid + Strong Base → Basic salt + H2O Strong Acid + Strong Base → Neutral salt + H2O Weak Acid + Weak Base → Neutral salt + H2O 15 Reaction of Acids and Bases with each other www.mgschooledu.blogspot.com MG SCHOOL SCIENCE Chapter 2 Acids, Bases and Salts
- 16. Metallic oxides are basic in nature. Example: CaO, MgO are basic oxides. Metallic Oxide + Acid → Salt + H2O CaO + 2HCl → CaCl2 + H2O Non-metallic oxides are acidic in nature. Non-metallic Oxide + Base → Salt + H2O CO2 + Ca(OH)2 → CaCO3 + H2O 16 Reaction of Metallic Oxides with Acids Reaction of Non-Metallic Oxides with bases www.mgschooledu.blogspot.com MG SCHOOL SCIENCE Chapter 2 Acids, Bases and Salts
- 17. 17 (i) Acid + Metal Carbonate → Salt + CO2 + Water (ii) Acid + Metal → Salt + H2 (iii) Acid + Metal Hydrogen Carbonate → Salt + CO2 + H2O (iv) Acid + Metallic oxide → Salt +H2O (v) Acid + Base → Salt + H2O (i) Base + Metal → Salt + H2 (ii) Base + Metal Carbonate → No Reaction (iii) Base + Metal Hydrogen Carbonate → No Reaction (iv) Base + Acid → Salt + H2O (v) Base + Non Metallic oxide → Salt + H2O Reaction of Acid Reaction Of Base www.mgschooledu.blogspot.com MG SCHOOL SCIENCE Chapter 2 Acids, Bases and Salts
- 18. • All acids have H+ ions in common. • Acids produce H+ ions in solution which are responsible for their acidic properties. • All bases have OH- (hydroxyl ions) in common • Acids produce H+ ions in presence of water. • H+ ions cannot exist alone, they exist as H3O+ (hydronium ions). • H+ + H2O → H3O+ • HCl + H2O → H3O+ + Cl- • Bases when dissolved in water gives OH− ions. • NaOH + H2O → OH- + Na+ • Bases soluble in water are called alkali. Note: While diluting acids, it is recommended that the acid should be added to water and not water to acid because the process of dissolving a acid or a base in water is highly exothermic. 18 Similarities between all Acids and all Bases Acid or Base in Water Solution www.mgschooledu.blogspot.com MG SCHOOL SCIENCE Chapter 2 Acids, Bases and Salts
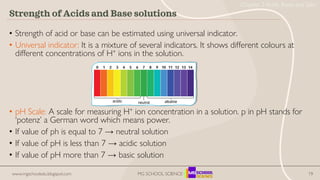
- 19. 19 • Strength of acid or base can be estimated using universal indicator. • Universal indicator: It is a mixture of several indicators. It shows different colours at different concentrations of H+ ions in the solution. • pH Scale: A scale for measuring H+ ion concentration in a solution. p in pH stands for ‘potenz’ a German word which means power. • If value of ph is equal to 7 → neutral solution • If value of pH is less than 7 → acidic solution • If value of pH more than 7 → basic solution Strength of Acids and Base solutions www.mgschooledu.blogspot.com MG SCHOOL SCIENCE Chapter 2 Acids, Bases and Salts
- 20. Organic Acids and their Sources 20 Vinegar Acetic acid Curd Lactic acid Oranges Citric acid Lemons Citric acid Tamarind Tartaric acid Ant sting Formic acid Apples Malic acid tomotoes Oxalic acid www.mgschooledu.blogspot.com MG SCHOOL SCIENCE Chapter 2 Acids, Bases and Salts