Nitrate and Nitrite poisoning
- 1. Nitrates and Nitrites Poisonig Abdulkarim nazar jabar & Nadeen Alaa Al- sheikh
- 2. If I can ease one life the aching, Or cool one pain, I shall not live in vain. —Emily Dickinson
- 3. Nitrate and Nitrite Poisoning Introduction : An inorganic chemicals that is highly soluble in water. Microbes break down animal’s and human’s organic wastes (in soil and water) into ammonia, which then oxidizes into nitrite and nitrate. Nitrates also occur naturally : in the environment, in mineral deposits, soil, seawater, freshwater systems, and the atmosphere. The body also makes approximately 62 mg / day of nitrate in addition to what is ingested. Infection and illness can cause the body to produce even greater levels of nitrate. Attention has been drawn to the possible danger of herbicide’s (2,4- dichlorophenoxyacetic acid) use during periods of drought , may results in accumulation of Nitrate , Air-dried leaves from plants sprayed with 2,4-D were found to contain 4.5% KNO3 (minimum lethal concentration in animal fodder) (Stahler and Whitehead 1950), while the untreated plants contain 0.22%.
- 4. Uses of Nitrite and Nitrate : Artificail manures and fertilisers (sodium , potassium and ammonium nitrate) As a Preservative (in Some meats and meat products contain sodium nitrate and / or sodium nitrite as preservatives). Medications (Nitroglycerine , Amyl nitrite , nitroprusside ..) As a Color enhancement of processed meats (although the amount added to these products have been substantially reduced from the levels once used). In Dynamites ( contain Ammonium Nitrate) Industry (Purified potassuim nitrate for glass making).
- 5. Etiology and source of exposure : Major sources of nitrate toxicity in drinking water include fertilizers, sewage and animal manure. *Nitrite can also be formed chemically in distribution pipes by Nitrosomonas bacteria during stagnation of (nitrate- containing and oxygen-poor) drinking-water in galvanized steel pipes. Food is usually the major source of nitrate exposure. Ingestion of up to 250 mg / day of nitrate has been reported for people whose diets consist mainly of food from vegetable sources.
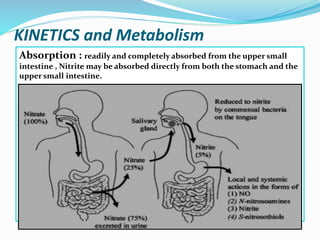
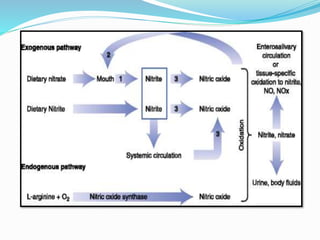
- 6. KINETICS and Metabolism Absorption : readily and completely absorbed from the upper small intestine , Nitrite may be absorbed directly from both the stomach and the upper small intestine.
- 7. Infants convert approximately double, or 10% of ingested nitrate to nitrite compared to 5% conversion in older children and adults. Nitrite in the bloodstream is involved in the oxidation of haemoglobin (Hb) to methaemoglobin (metHb) , the Fe2+ present in the haem group is oxidized to its Fe3+ form, and the remaining nitrite binds firmly to this oxidized haem. The Fe3+ form does not allow oxygen transport, owing to the strong binding of oxygen (Jaffé, 1981; United States National Research Council, 1995). Therefore, methaemoglobinaemia can lead to cyanosis.
- 9. Acute and Chronic effect of Nitrates 1.Short term effect (acute): Development of methaemoglobinemia (an excess of methemoglobin in the blood) that cannot carry oxygen. High conc. can result in a temporary blood disorder in infants called methemoglobinemia, commonly called " blue baby syndrome". In severe, untreated cases, brain damage and eventually death can result from suffocation due to lack of oxygen. Predisposing factors : Age (infants 6 months of age are considered to be the most sensitive population) Pregnant women (due to a natural increase in methemoglobin levels during the later stage of pregnancy beginning around the 3oth week).
- 10. Individuals with digestive difficulties (due to reduced stomach acidity are also at higher risk). 2.Chronic effect (carcinogenic effect): It is believed that after nitrate is converted to nitrite in the body, it can react with certain amine- containing substances found on food to form Nitrosamines, which are known to be potent cancer causing chemicals. Nitrosamine formation is inhibited by antioxidants that may be present in food such as Vitamin C and Vitamin E. 3.Reproductive / Developmental Effects: A study in which an association between nitrate levels and an increase in neural tube defects was observed.
- 11. Clinical symptoms Irritability, lack of energy, headache, dizziness, vomiting, Diarrhea. labored breathing. blue – gray or pale purple coloration to areas around the eyes, mouth, lips, hands and feet. cyanosis (blue skin) of limbs / trunk, weakness, and rapid heart rate , due to methaemoglobinemia. Sever Methaemoglobinemia : CNS depression. brief loss of consciousness, shock, convulsions, coma. Irregular heart beats. Death occur when methaemoglobin levels exceed 50%.
- 12. Nitrate medications (Nitroprusside) cause hypotension due to excesive vasodilation. Detection of Nitrates in drinking water : The US. Environmental Protection Agenc (EPA) has set an enforceable standard called a maximum contaminant level ( MCL) for nitrates at 10 ppm and for nitrite at 1 ppm in drinking water. Treatment : Most healthy people over 6 months of age have internal mechanisms efficient at removing nitrate from the body. Therefore, treatment of exposure to nitrates and nitrites is typically not required for mild and moderate cases. Hospital treat extreme cases of exposure by applying 100% oxygen and methylene blue. The most important step in treating persons with nitrates poisoning is to determine and remove the source of the nitrate.
- 13. Prevention You should avoid exposure to water, soil, or food contaminated with high levels of nitrates and nitrites. If you have well water that comes from areas that contain large amounts of nitrogen- containing fertilizers, you should monitor the water closely. _____________________________________________________________ References Agency for Toxic Substances and Disease Registry (ATSDR). 2004ealth and Human Services, Public Health Service.. Interaction Profile for: cyanide, fluoride, nitrate, and uranium. Atlanta, GA: US . Department of Public Health and Human Services, Public Health Service. Agency for Toxic Substances and Disease Registry (ATSDR) 2007. Case Studies in Environmental Medicine, Nitrate/ Nitrite Toxicity. Atlanta, GA: US. Department of public Agency for toxic Substances and Diseases Registry, U.S. Department of Health and Human Services in Atlanta, GA. 2015Toxicological Profile for Nitrate and Nitrite . Casarett and Doull's Toxicology: The Basic Science of Poisons, Sixth Edition. Klaassen, C.D., ed. Mc- Graw – Hill Publishing Co., 20012. Consumer fact sheet on: nitrates / nitrites, U.S., EPA, Office of Water. Revised: January, 1998. Nathan DM ; Siegal AJ, Bunn HF. 1977. Acute methemoglobinemia and hemolytic anemia with phenazopyridine. Arch Intern Med: 137 (11): 1636-1638. Nitrate and nitrite in drinking water. National Research Council, National Academy of Sciences. National Academy Press. 1995. Toxicological information on nitrate. Integrated Risk. Information System. U.S. EPA , office of Health and Environmental Assessment; Last review: August , 1990.