Chapters
Do you know? There are more than 100 elements on this Earth and we are not even counting the isotopes. There are so many elements that making a list of them became a requirement. A long time ago, a scientist named Mendeleev made a list of them. Today, just a simple list made our lives easier. It is not a list, it is more of a table, and we call it the periodic table. In this resource, we will give you a brief introduction to the periodic table.

What is a Periodic Table?
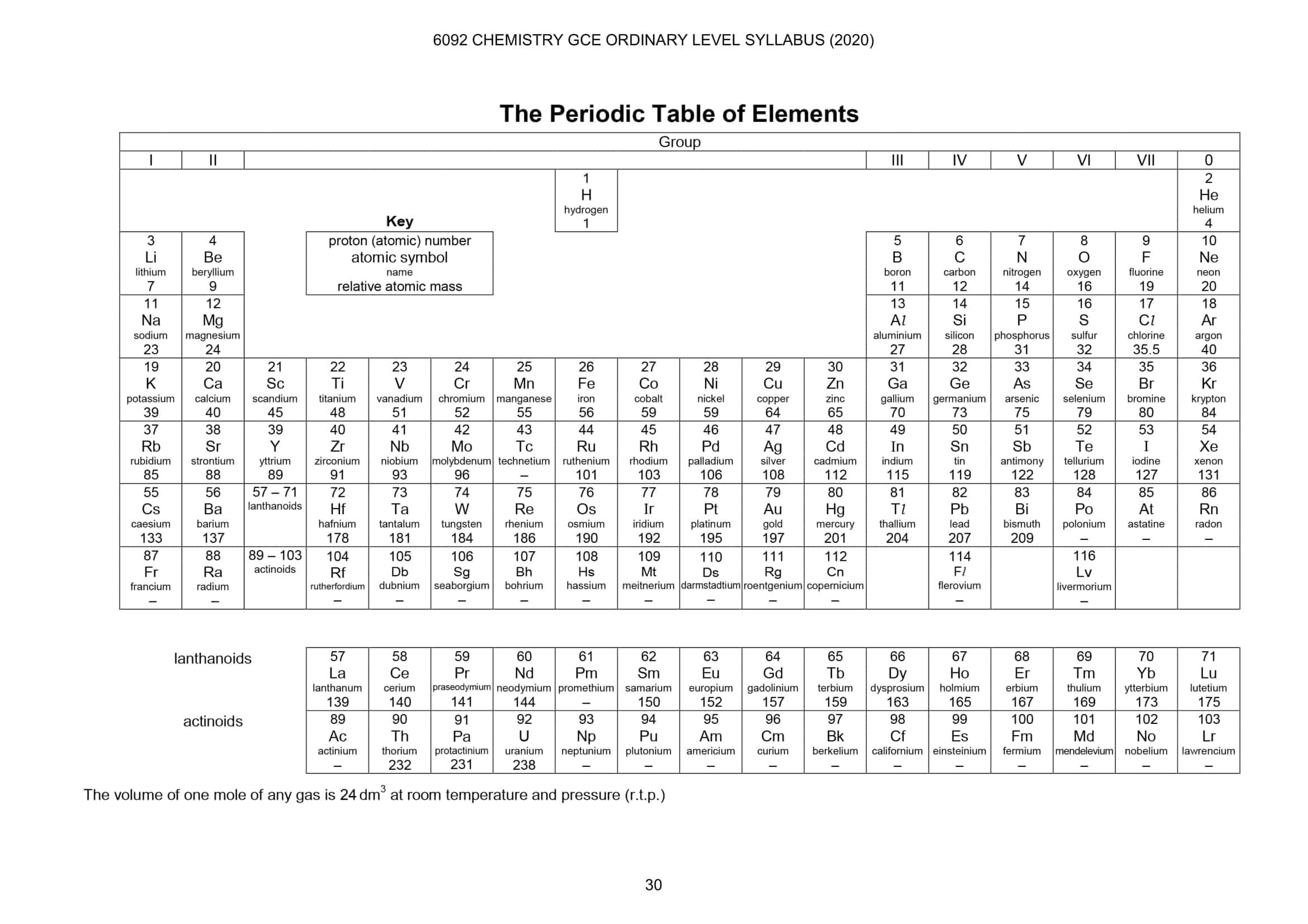
A periodic table is an arrangement of all elements with respect to their increasing proton numbers. Like in the library, you see how books are arranged according to their codes or names, etc. In the same way, the periodic table is also arranged but according to an increase in proton number. A periodic table isn't just a list of elements, there is a lot going on behind it. There is so much that you can learn from a periodic table. We will discuss in upcoming resources how you can learn about other properties just by looking at the periodic table. A periodic table is divided into two parts, periods and groups.
What are Groups?
The vertical column of a periodic table is called a group. There are a total of eight groups in a periodic table and each group is numbered using a roman numeric system. In the middle, you will see that some elements are not grouped. They are called transition elements and they are not grouped. The best way to identify the group is that you should look at the top, if the group has a roman number on it then it is a group.
The groups are numbered from I to VII. In a periodic table, the last group is either called group 0 or group VIII. Don't get confused about group 0 and group VIII, both are the same. The groups run from top to bottom. Moreover, the groups tell about the valance electrons. For example, in group I, all the elements will have one electron in their valance shell. The shell will vary as you move up or down but the number of electrons in the valance shell will remain the same. The numbering on the group shows the number of electrons in the valance shell. Suppose you are asked to tell the valance electrons of carbon. All you need is to locate the group to which carbon belongs. Carbon belongs to group V therefore, carbon has five electrons in its valance shell.
What Are Periods?
Periods are the horizontal rows of elements in a periodic table. There are seven periods in a periodic table, unlike groups, periods are not numbered. In some periodic tables, you might find periods also numbered but that is done for the reader's ease, officially, periods are not numbered in a periodic table. Like groups, periods also provide information and that is the number of shells that electrons have accommodated. For example, in the 3rd period, the number 3 means that there are three shells on which electrons are accommodated.
Imagine, you are asked to find the number of shells that electrons have occupied in an atom of chlorine this time. You can get this answer in two ways. Either you draw the whole atomic structure just to find the number of shells utilized or you can just check in which period is chlorine and that is your answer. Since we are talking about the periodic table here, we will use the second method. Chlorine exists in the third period (we found this by counting the rows starting from hydrogen), therefore, the number of shells utilized in chlorine is 3.
What are Transition Elements?
Do you see elements between group 2 and group 3? They are not categorized in groups, in fact, they are separate elements because they exhibit different chemical and physical properties. They are called transition elements.
How To Locate Elements From Periodic Table?
Learning how to locate the periodic table is just the beginning. This is fundamental chemistry, which means that you should know how to read a periodic table before you move to complex chemistry. As mentioned before, elements are listed according to their proton numbers in the periodic table, the best way to locate any element on a periodic table is through proton numbers.
For example, your teacher asked you to find the element whose proton number is 80. The best way to locate this element is to check the proton number of the periods. If the proton number is greater than the proton number that you are looking for that means your element is in the previous period. We are looking for an element having proton number 80 in a periodic table. Start checking proton numbers of the first element of a period and compare whether it is greater than 80 or not. If it is less than 80 then check the proton number for the next period. The key is to find the proton number greater than 80 and if you find that period that means the previous period contains your element. Therefore, in period 7, the proton number of the first element (which is Francium) is 87. This means that our element, whose proton number is 80, is in period number 6. Check all the elements in period number 6. Did you find it? If your answer is Mercury (chemical symbol: Hg) that means you understood how to read elements from the periodic table.
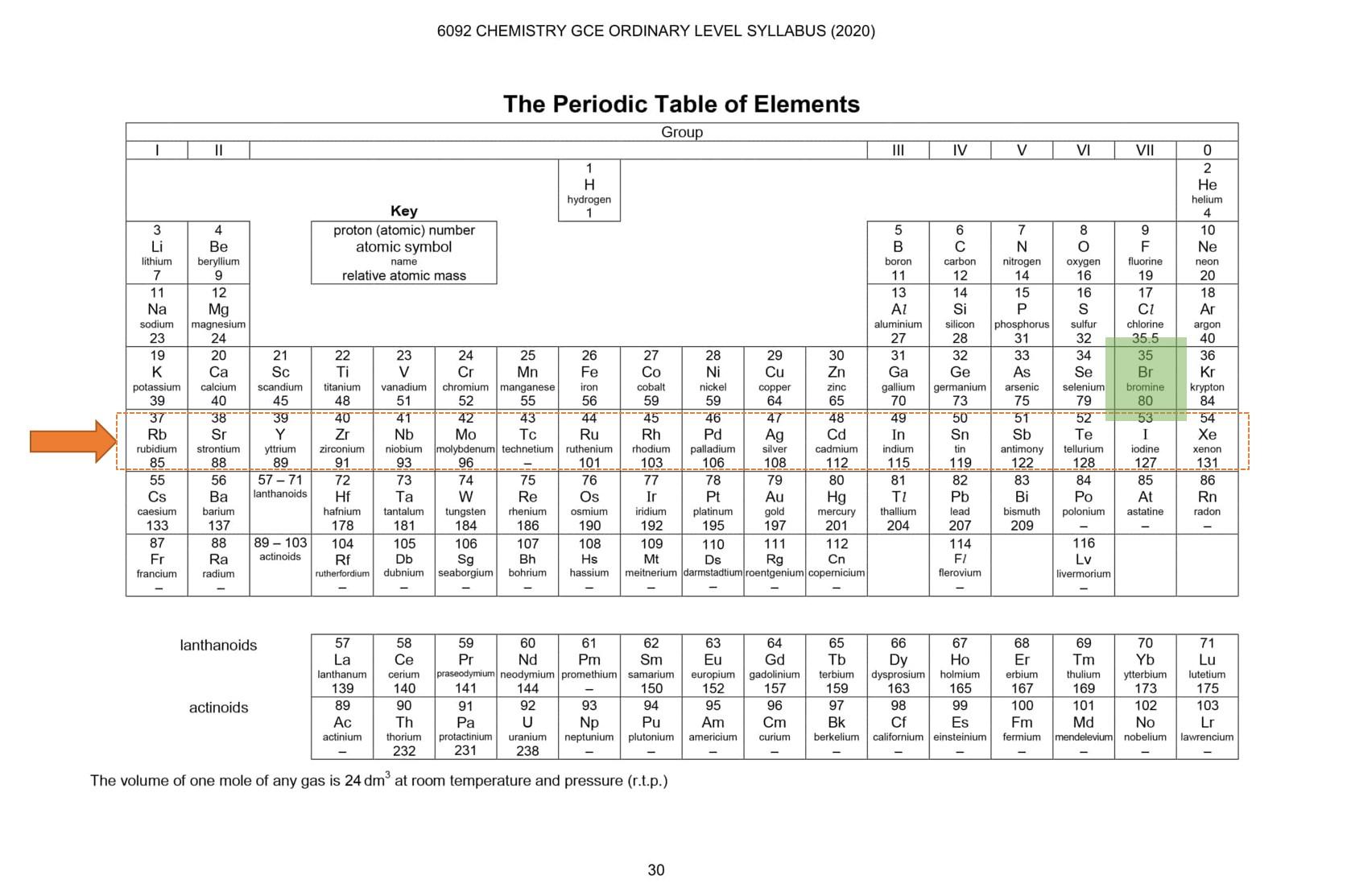
Let's do another example, find the element Iodine. The proton number of iodine is 53. Take your time and find the element in the periodic table. If you are unable to find it, allow us to help you. Check period number 6, the first element in period 6 has a proton number of 55 and 55 is greater than 53 therefore, iodine is in the previous period. Check all the elements in that period and locate iodine (chemical symbol: I). The key is to find the right period number and once you locate it that means you are very near to your answer.
How To Read Elements in a Periodic Table?
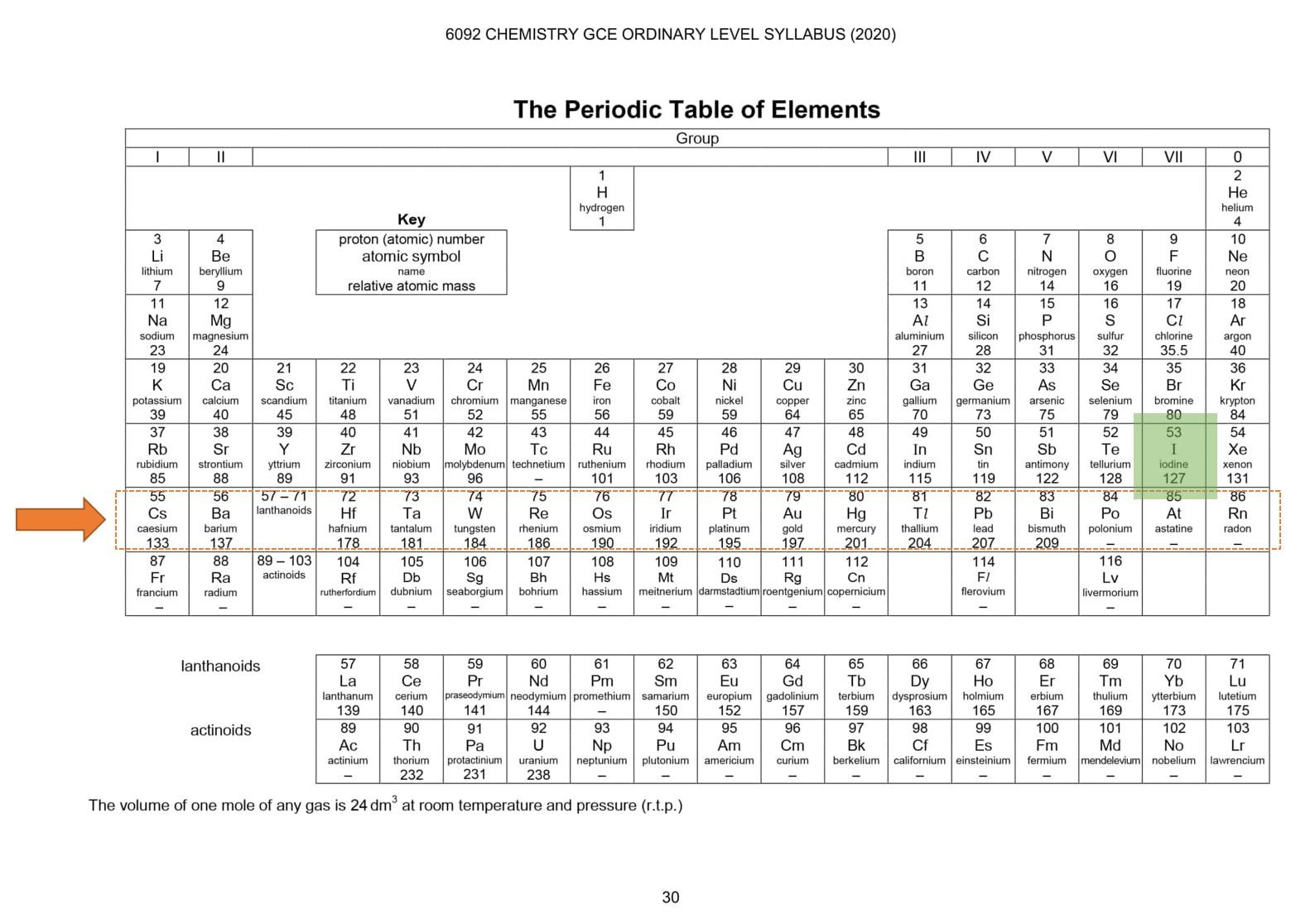
If you are able to locate elements from a periodic table then reading them is pretty easy. The first step is to locate the element in the periodic table. For example, you located the calcium element in the periodic table. You can learn about its group by looking at the top numbering row and you can learn about its period (by counting the rows). Calcium is in group 2 and period 4. What does this tell you? It tells you that calcium has 4 shells occupied and the last shell has 2 electrons. Remember we told you group tells about valance electrons and period tells about the number of shells utilized? We already found the period and group of the calcium and using this knowledge, we just figured out the atomic structure of calcium.
Here is another example for you to try. Find the valance electrons and number of shells utilized of argon. The first part is to locate it. Once located, learn about its period and group. Argon belongs to group 8 and is in the 4th period. This means argon has 4 shells occupied and the valance shell contains 8 electrons.
Conclusion
In this resource, we focused on a brief introduction to the periodic table and how to locate and read information from the periodic table. This is very important since you will be making chemical equations later and to make such equations, you definitely require a periodic table. In upcoming resources, we will teach you about the history of the periodic table and we will discuss some groups in detail as mentioned in the GCSE Chemistry course.












Was very educative and help. Gave me a broader understanding of the topic